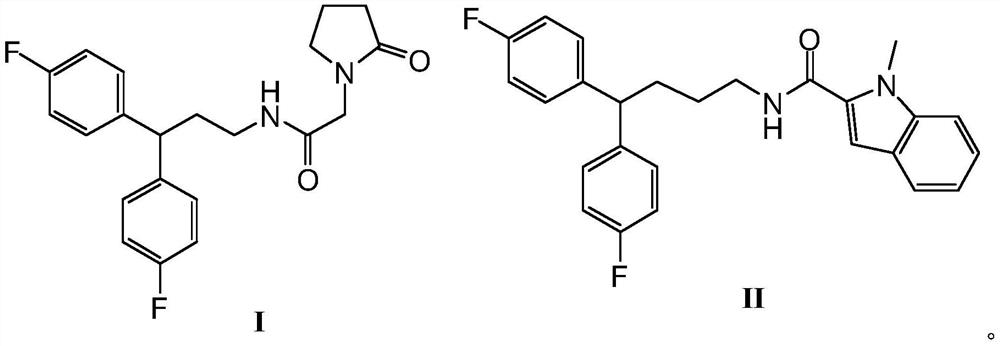
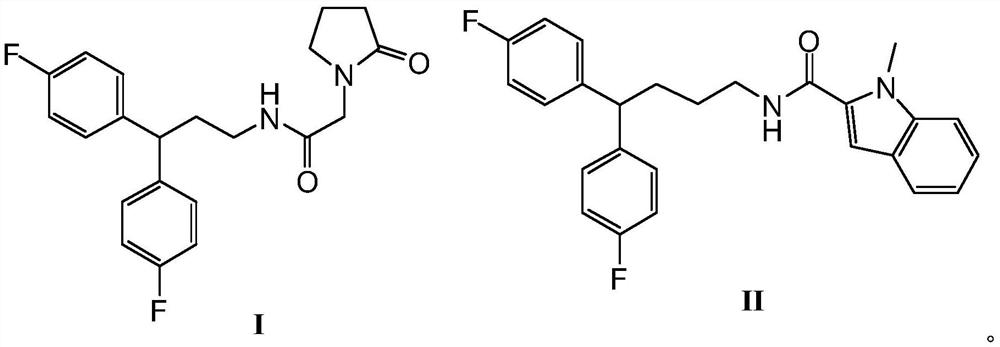
Anti-prostate cancer compound and preparation method thereof
An anti-prostate cancer, prostate cancer technology, applied in anti-tumor drugs, organic chemistry, drug combination and other directions, can solve the problems of low drug activity, large toxic and side effects, etc., achieves simple preparation method, mild process conditions, and is suitable for large-scale production. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
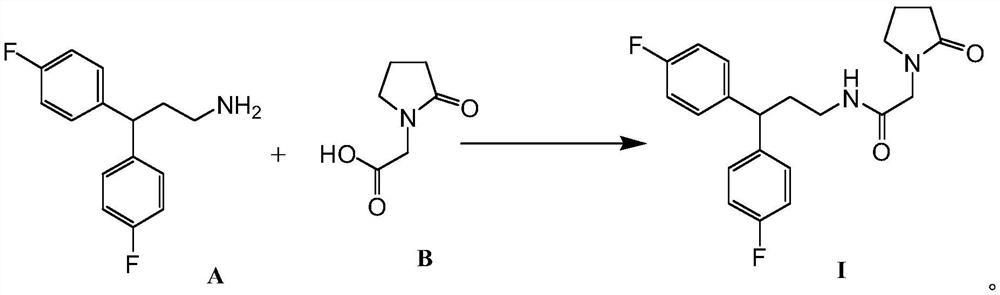
[0018] The preparation of formula I compound, synthetic route is as follows:
[0019]
[0020] Specific steps:
[0021] Add 1.52g of compound B, 10mL of tetrahydrofuran and 10mL of thionyl chloride into a 50mL single-port reaction flask with magnetic stirring, keep stirring, and reflux for 4 hours. Excess thionyl chloride was removed under reduced pressure, 12 mL of a tetrahydrofuran solution containing 1.65 g of compound A and 1 g of 4-(N,N-dimethyl)pyridine were added, and stirred at room temperature for 10 hours. The reactant in the reaction bottle was placed in a 100mL separatory funnel, 25mL ethyl acetate was added, and 5% Na 2 CO 3 Wash twice with 25 mL of aqueous solution, then twice with 20 mL of water, and finally once with 10 mL of saturated NaCl aqueous solution. The organic layer was separated, dried over anhydrous sodium sulfate, filtered, concentrated, purified by column chromatography, and concentrated again to obtain the compound of formula I (1.28 g). 1...
Embodiment 2
[0023] The preparation of formula II compound, synthetic route is as follows:
[0024]
[0025] Specific steps:
[0026] Add the compound of formula 1.20gb, 10mL tetrahydrofuran and 10mL thionyl chloride into a 50mL single-port reaction flask with magnetic stirring, keep stirring, and reflux for 4 hours. Excess thionyl chloride was removed under reduced pressure, 12 mL of a tetrahydrofuran solution containing 1.10 g of the compound of formula a and 1 g of 4-(N,N-dimethyl)pyridine were added, and stirred at room temperature for 10 hours. Transfer the reactants in the reaction bottle to a 100mL separatory funnel, add 25mL ethyl acetate to dilute, and dilute with 5% Na 2 CO 3 Wash twice with 25 mL of aqueous solution, then twice with 20 mL of water, and finally once with 10 mL of saturated NaCl aqueous solution. The organic layer was separated, dried over anhydrous sodium sulfate, concentrated after filtration, and purified by column chromatography to obtain the compound of...
Embodiment 3
[0027] Example 3 Biological Example
[0028] Use the MTT method to detect the activity of the above-mentioned compounds of formula I and formula II inhibiting prostate cancer DU145 cells and prostate cancer PC3 cells. For details, see CN 104098479 A. The IC50 of the compounds of formula I and formula II killing prostate cancer DU145 cells and prostate cancer PC3 cells is shown in the table 1.
[0029] The killing effect (IC50 / μM) of each compound of table 1 on prostate cancer cells
[0030]
[0031] From the results in Table 1, it can be seen that the compound of formula I of the present invention has a good inhibitory effect on prostate cancer cells, whether it is DU145 cells or prostate cancer PC3 cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com