Method for synthesizing aromatic monofluoromethylthio compounds by one-pot method
A monofluoromethylthio and aromatic technology, applied in organic chemistry, thioether preparation, etc., can solve the problems of low substrate expansion ability, few types of S-containing substrates, difficult operation and treatment, etc., and achieve high conversion rate , environment-friendly, and easy conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
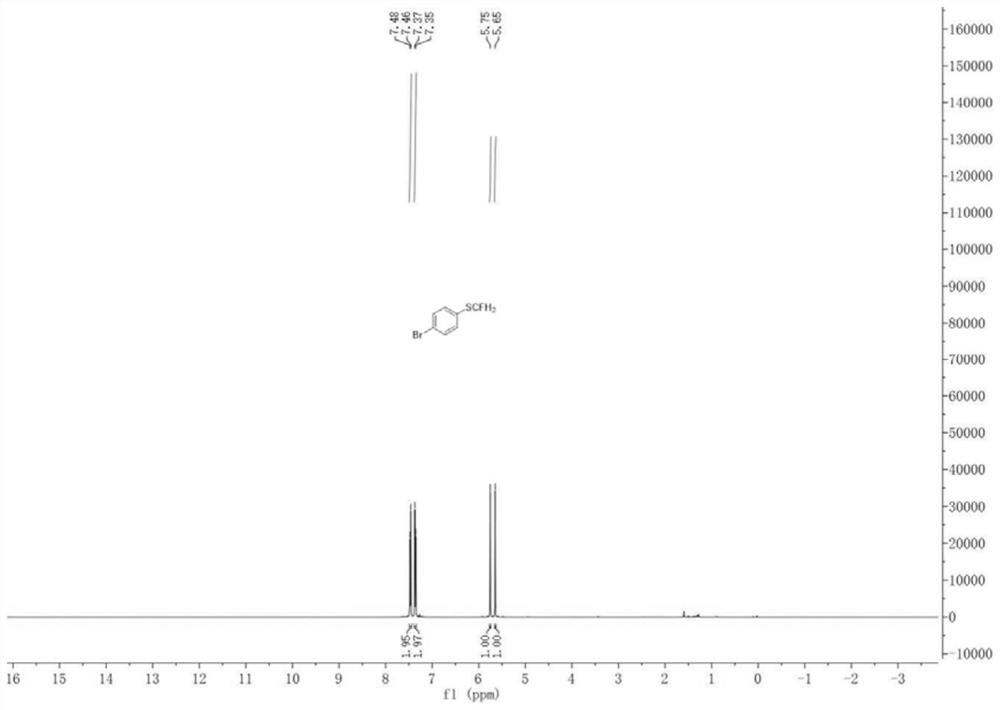
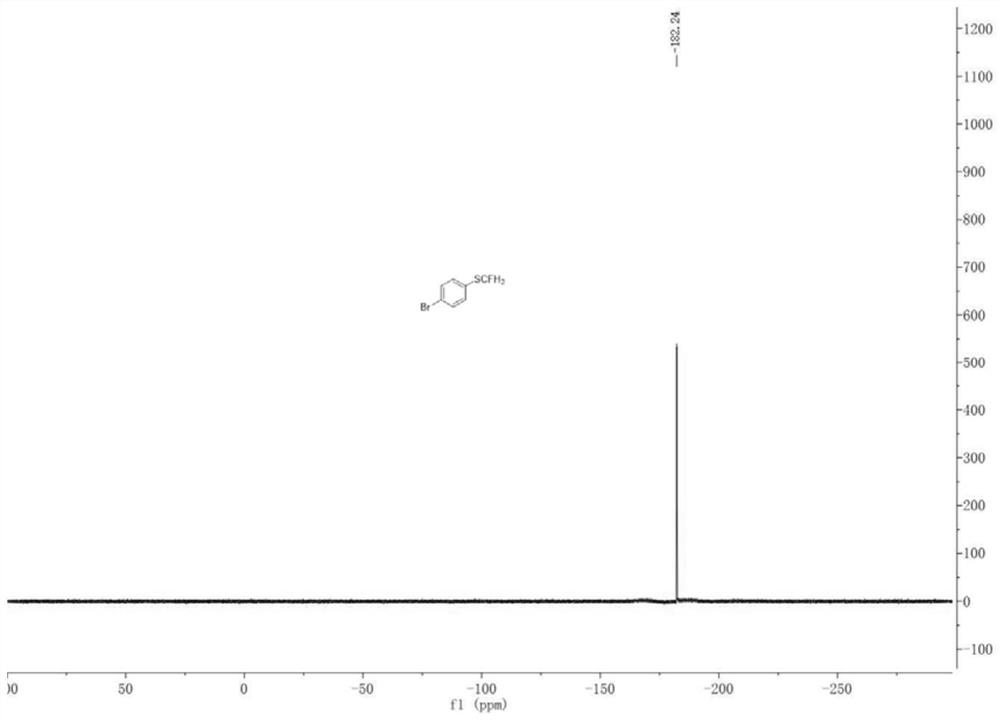
[0049] Add a magnetic stir bar, 4-bromoaniline (0.5 mmol), HBF to the dry pressure. 4 (1 mmol), tert-butyl nitrite (1 mmol) and anhydrous acetonitrile (2 ml), the reaction was placed in an ice bath for 2 h. After the reaction was completed, CuCl (0.05 mmol) was added, CuCl 2 (0.05 mmol), 1,10-phenanthroline (0.05 mmol) and KSCN (0.75 mmol), and stirred in an ice bath for 3 h. After the reaction is over, add KOH (5mmol) and ICFH to the system 2 (1 mmol) in an ice bath to continue the reaction for 7 h. After the reaction was finished, the reaction solution was filtered after returning to room temperature, the filtrate was dried over anhydrous sodium sulfate and the solvent was removed under reduced pressure, and the crude product was through column chromatography (eluent was ethyl acetate and petroleum ether mixed solution, the two The volume ratio is 1:19) to obtain 98.35 mg of 1-monofluorothiomethyl-4-bromobenzene with a yield of 89%.
[0050] 1-Monofluorothiomethyl-4-brom...
Embodiment 2
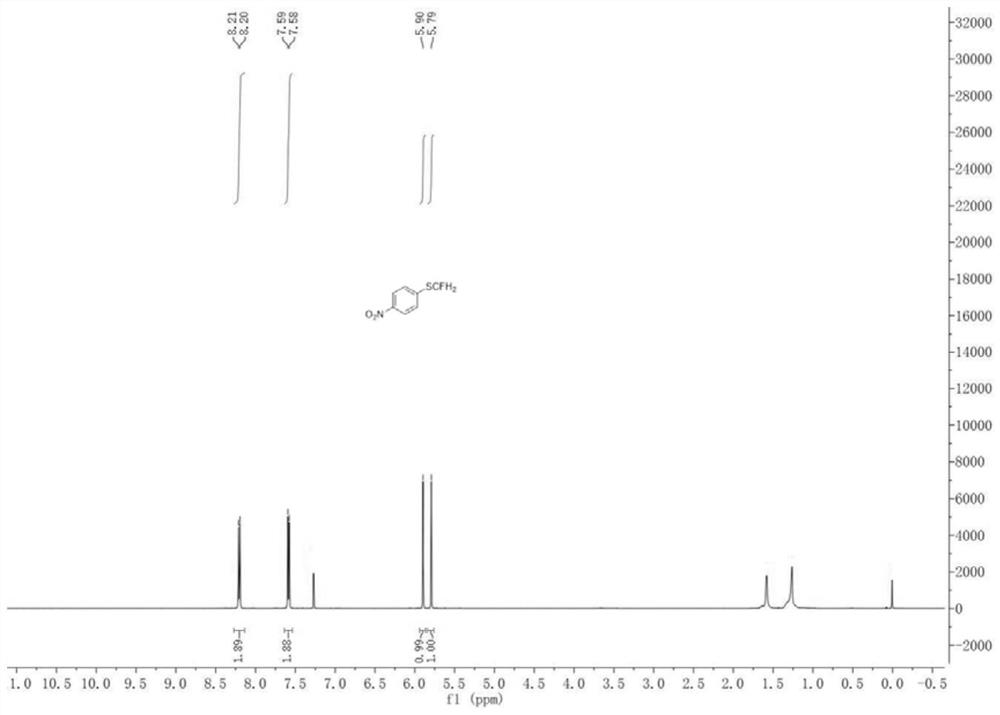
[0053] Add a magnetic stir bar, 4-nitroaniline (0.5 mmol), HBF to the dry pressure. 4 (1 mmol), tert-butyl nitrite (1 mmol) and anhydrous acetonitrile (2 ml), and the reaction was placed in an ice bath for 2 h. After the reaction was completed, CuCl (0.05 mmol) was added, CuCl 2 (0.05 mmol), 1,10-phenanthroline (0.05 mmol) and KSCN (0.75 mmol), and stirred in an ice bath for 3 h. After the reaction is over, add KOH (5mmol) and ICFH to the system 2 (1 mmol) in an ice bath to continue the reaction for 7 h. After the reaction is finished, the reaction solution is filtered after returning to room temperature, the filtrate is dried over anhydrous sodium sulfate and the solvent is removed under reduced pressure, and the crude product is through column chromatography (eluent is ethyl acetate and petroleum ether mixed solution, the two The volume ratio is 1:19) to isolate 86.02 mg of 1-monofluorothiomethyl-4-nitrobenzene with a yield of 92%.
[0054] 1-Monofluorothiomethyl-4-nitro...
Embodiment 3
[0057] Add a magnetic stir bar, 4-cyanoaniline (0.5 mmol), HBF to the dry pressure. 4 (1 mmol), tert-butyl nitrite (1 mmol) and anhydrous acetonitrile (2 ml), and the reaction was placed in an ice bath for 2 h. After the reaction was completed, CuCl (0.05 mmol) was added, CuCl 2 (0.05 mmol), 1,10-phenanthroline (0.05 mmol) and KSCN (0.75 mmol), and stirred in an ice bath for 3 h. After the reaction is over, add KOH (5mmol) and ICFH to the system 2 (1 mmol) in an ice bath to continue the reaction for 7 h. After the reaction is finished, the reaction solution is filtered after returning to room temperature, the filtrate is dried over anhydrous sodium sulfate and the solvent is removed under reduced pressure, and the crude product is through column chromatography (eluent is ethyl acetate and petroleum ether mixed solution, the two The volume ratio is 1:19) to isolate 76.82 mg of 1-monofluorothiomethyl-4-cyanobenzene with a yield of 92%.
[0058] 1-Monofluorothiomethyl-4-cyano...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com