Clinical research time schedule generation method and system, and equipment
A technology for generating systems and time, applied in electronic clinical trials, medical care resources or facilities, instruments, etc., can solve problems such as inability to determine clinical tasks and scheduling, and consumption of CRC work energy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
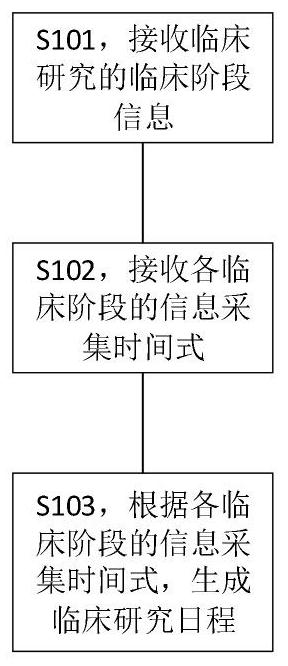
[0121] Specifically, as figure 1 As shown, the present invention provides a method for generating a clinical research schedule, comprising the following steps:
[0122] S101, receiving clinical stage information of clinical research;
[0123] In the specific implementation process, the clinical stage information includes various stages of the clinical research project, including but not limited to: screening period, treatment period, unplanned visit, post-treatment visit (EOT visit), safety follow-up , long-term follow-up, etc. During the above clinical stage, the clinical coordinator (CRC) needs to collect information from the patient to realize the record of the patient's clinical situation. The content of the information collection includes, but is not limited to, informed consent, medical history collection, physical examination, physical examination, blood urine and stool examination, and the like.
[0124] As an optional embodiment, after receiving the clinical stage ...
Embodiment 2
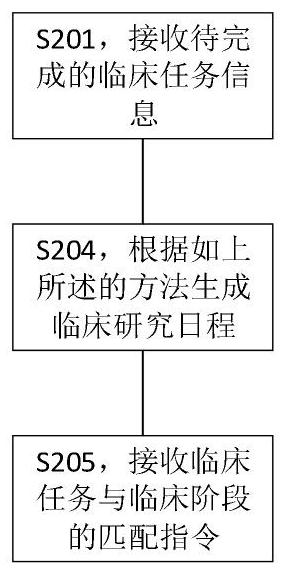
[0150] like figure 2 As shown, the present invention also provides a method for generating a clinical research time plan, comprising the following steps:
[0151] S201, receiving clinical task information to be completed;
[0152] In the specific implementation process, the creator of the clinical research time plan enters the clinical task information to be completed. In some embodiments, the clinical tasks to be completed include informed consent, qualification standard confirmation, medical history investigation, smoking status investigation, Physical examination, questionnaire filling, weight examination, height examination, electrocardiogram examination, hematology examination, biochemical examination, urinalysis, etc. The above tasks to be completed are clinical tasks that need to be completed one or more times in different stages of clinical research.
[0153] S204, generating a clinical research schedule according to the above method;
[0154] In the specific implemen...
Embodiment 3
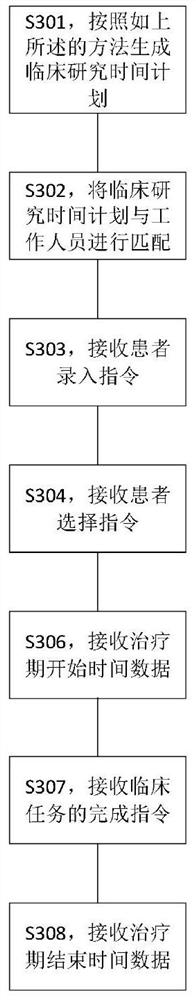
[0170] like image 3 As shown, the present invention also provides a progress recording method for a clinical research project, comprising the following steps:
[0171] S301, generating a clinical research time plan according to the above method;
[0172] During the specific implementation, any method as described in Example 2 was used to generate the clinical study time plan.
[0173] S302, match the clinical research time plan with the staff;
[0174] In the specific implementation process, the clinical research time plan generated in S301 is matched with the corresponding personnel in the system, and the staff member can obtain the corresponding clinical research time plan through a terminal device such as a mobile phone by means of account login, etc. The above acquisition method can be realized through a small program or APP of the mobile terminal. The above staff can be CRC.
[0175] S303, receiving a patient input instruction;
[0176] In the specific implementatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com