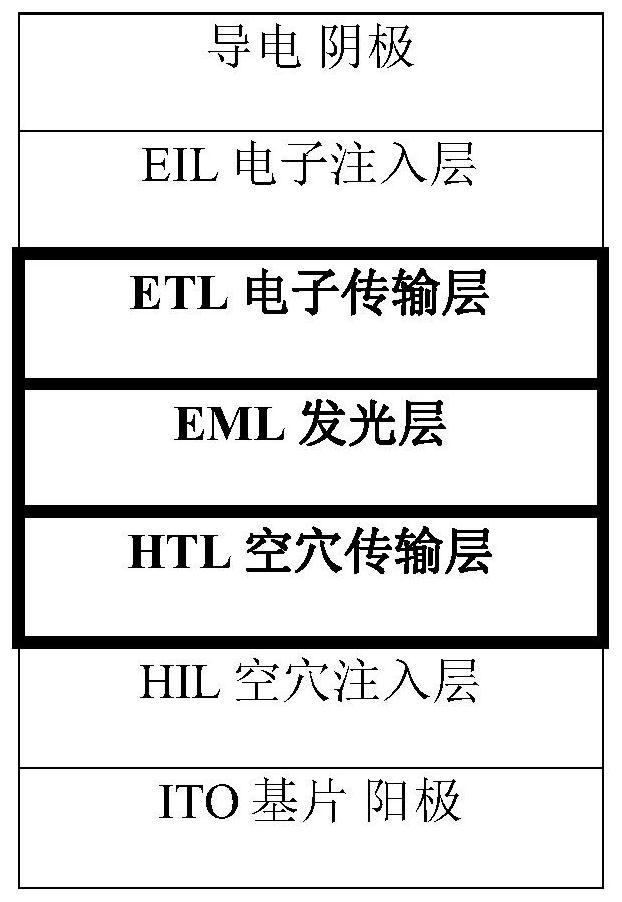
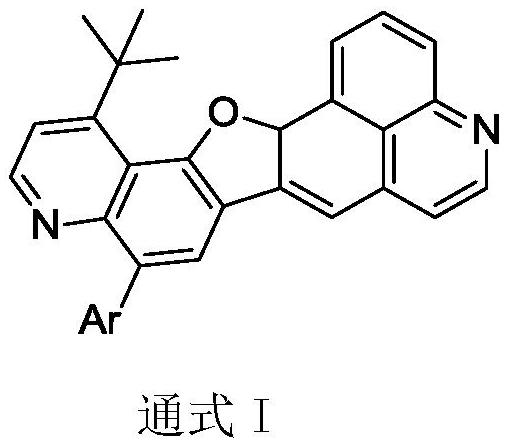
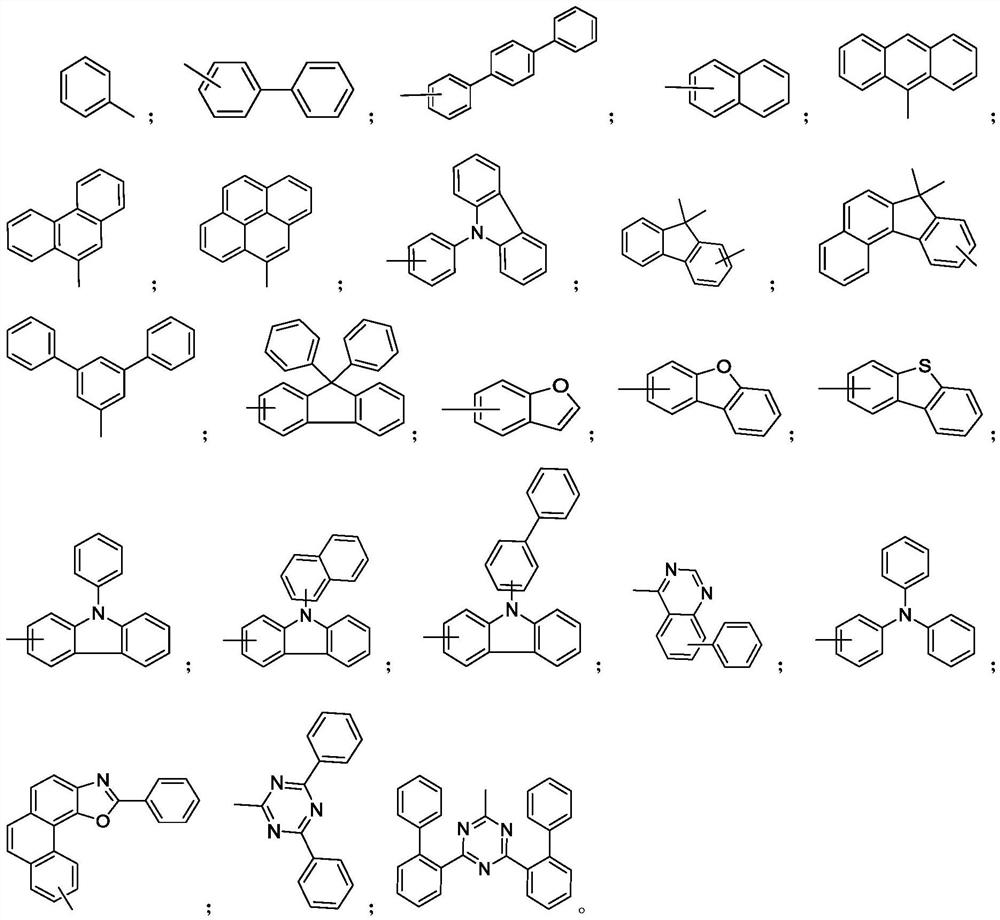
A kind of quinoline material containing benzofuran structure and its preparation method and application
A technology of benzofuran and quinoline, which is applied in the field of OLED materials, can solve the problems of poor luminous performance, high driving voltage, and low luminous efficiency, and achieve excellent performance, reduce driving pressure, and prolong working life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: A structural formula of compound A applied in the OLED field:
[0041]
[0042] The concrete synthetic route of compound A is:
[0043]
[0044] Preparation of Compound A-1: Weigh cyanuric chloride and 2-biphenylboronic acid according to the molar ratio of 1:2.5, add catalyst palladium acetate and tricyclohexylphosphine, the molar ratio of catalyst to cyanuric chloride is 0.1:1, The solvent is toluene, and the amount of the solvent is 10 times of the weight of cyanuric chloride. The reaction is carried out at 100° C. for 4 hours. According to HPLC detection, there is no remaining 2-biphenylboronic acid, and the reaction purity is 72.2%. Compound A-1 was obtained after water washing, concentration, column dissolution and recrystallization, with a yield of 54.5% and a HPLC purity of 96.4%. 1 H NMR (400 MHz, Acetonitrile-d3): δ7.44-7.66 (8H, m), 7.96-8.08 (8H, m), 8.20 (2H, d).
[0045] Preparation of compound A: Weigh compound A-1, intermediate I a...
Embodiment 2
[0046] Embodiment 2: A structural formula of compound B applied in the OLED field:
[0047]
[0048] The specific synthetic route of compound B is:
[0049]
[0050] Preparation of compound B: Weigh intermediate I, 9-bromo-11,11-dimethylbenzofluorene and sodium carbonate according to the molar ratio of 1:1.2:2.5, add catalyst tris(dibenzylideneacetone) dipalladium ( 0) and 4,5-bisdiphenylphosphine-9,9-dimethylxanthene, the molar ratio of the catalyst to the intermediate I is 0.01:1, sodium carbonate is made into an aqueous solution with a mass fraction of 40%, and the solvent It is toluene, the amount of solvent is 10 times of the weight of intermediate I, and the reaction is incubated at 75° C. for 5 h. According to HPLC detection, 0.2% of intermediate I remains, and the reaction purity is 90.2%. Compound B was obtained after hydrolysis, water washing, column passing and recrystallization, with a yield of 88.1% and a HPLC purity of 99.2%. 1 H NMR (400MHz, CDCl3): δ1.7...
Embodiment 3
[0051] Embodiment 3: A structural formula of compound C applied in the OLED field:
[0052]
[0053] The specific synthetic route of compound C is:
[0054]
[0055] Preparation of compound C: Weigh intermediate I, 2-bromo-9,9-diphenylfluorene and sodium carbonate according to the molar ratio of 1:1.1:2.5, add catalyst tris(dibenzylideneacetone)dipalladium(0) and 4,5-bisdiphenylphosphine-9,9-dimethylxanthene, the molar ratio of catalyst to intermediate I is 0.01:1, sodium carbonate is made into an aqueous solution with a mass fraction of 30%, and the solvent is toluene , the amount of solvent was 10 times the weight of intermediate I, and the reaction was incubated at 75° C. for 8 hours. HPLC detection showed that 0.01% of intermediate I remained, and the reaction purity was 91.2%. Compound C was obtained after hydrolysis extraction, water washing, column passing and recrystallization, with a yield of 87.8% and a HPLC purity of 99.5%. 1 H NMR (400MHz, CDCl3): δ1.62(9H,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com