Application of anti-CD47 monoclonal antibody to medicines used for treating acute lymphocytic leukemia cells with amycin resistance
A technology of acute lymphocytes and leukemia cells, which is applied in the field of medicine and can solve problems that have not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
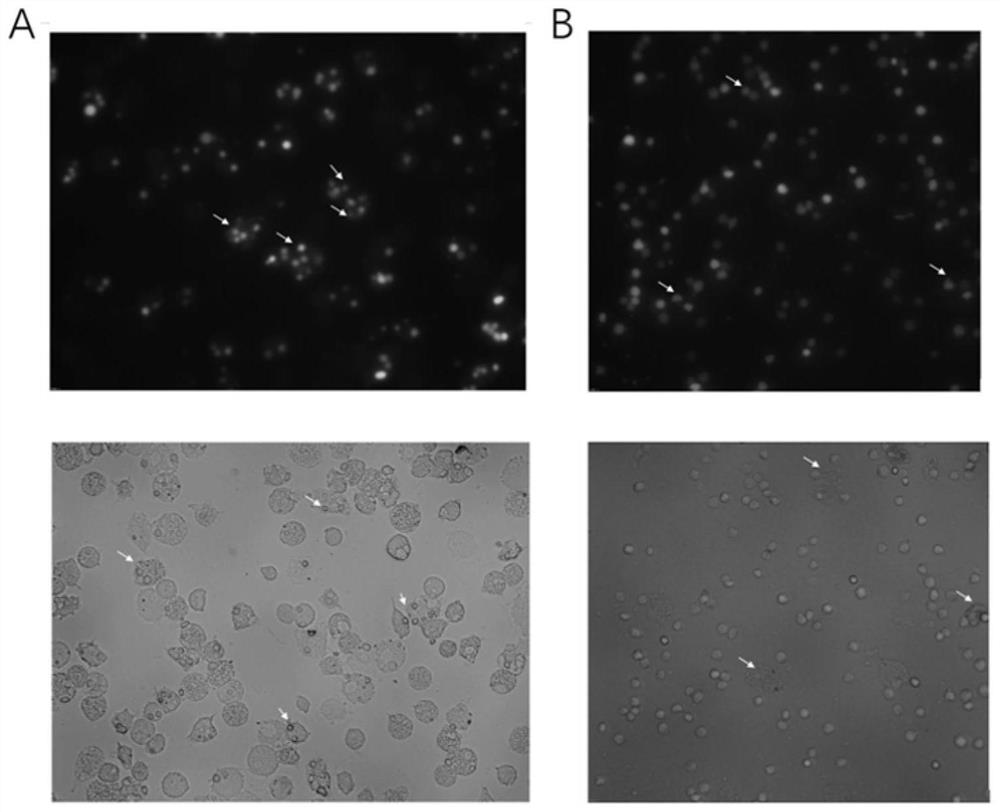
[0015] Example 1: In vitro detection of antibody-dependent cell-mediated phagocytosis (ADCP)
[0016] PBMC-derived macrophages were prepared from healthy donors, incubated with trypsin / EDTA (Gibco) for 3–5 min and scraped gently to obtain macrophages. 5.5×10 4 macrophages per well in a 24-well plate. Tumor cells were labeled with the standard dye 3 μM carboxyfluorescent succinimidyl ester (CFSE) according to standard protocols. After the macrophages were incubated in serum-free IMDM for 2-4 hours, 2.5×10 5 CFSE-labeled live tumor cells. Add 10-15 μg / mL anti-CD47 monoclonal antibody (Bio X Cell, USA), and incubate at 37°C for 2-4 hours. After incubation, the microwells were thoroughly washed 3-5 times with PBS and subsequently imaged with a fluorescence microscope. The phagocytosis index was calculated according to the number of effector cells (macrophages) that phagocytized CFSE-positive tumor cells. Analysis of results by fluorescence microscopy showed that anti-CD47 mo...
Embodiment 2
[0017] Example 2: In vivo effect of anti-CD47 monoclonal antibody against doxorubicin-resistant acute lymphoblastic leukemia systemic mouse model
[0018] 3-4 weeks old NOD / SCID mice were selected, and the doxorubicin-resistant acute lymphoblastic leukemia cell line Nalm6-Luc / ADR was used to establish a systemic B-ALL drug-resistant mouse model. First, a doxorubicin-resistant human B-ALL cell line Nalm6-Luc / ADR expressing luciferase in logarithmic growth phase was isolated. Count 3 x 10 4 Cells were injected into NOD / SCID mice through the tail vein. After the mouse model was established successfully, they were divided into PBS control group and anti-CD47 monoclonal antibody treatment group, with 10 mice in each group, and the dosage of anti-CD47 monoclonal antibody was 100ug / Only the control group was treated with PBS. Administration for 3 days, observation time 14 days. Mouse experiments were performed according to the guidelines for animal experiments of Fujian Medical U...
Embodiment 3
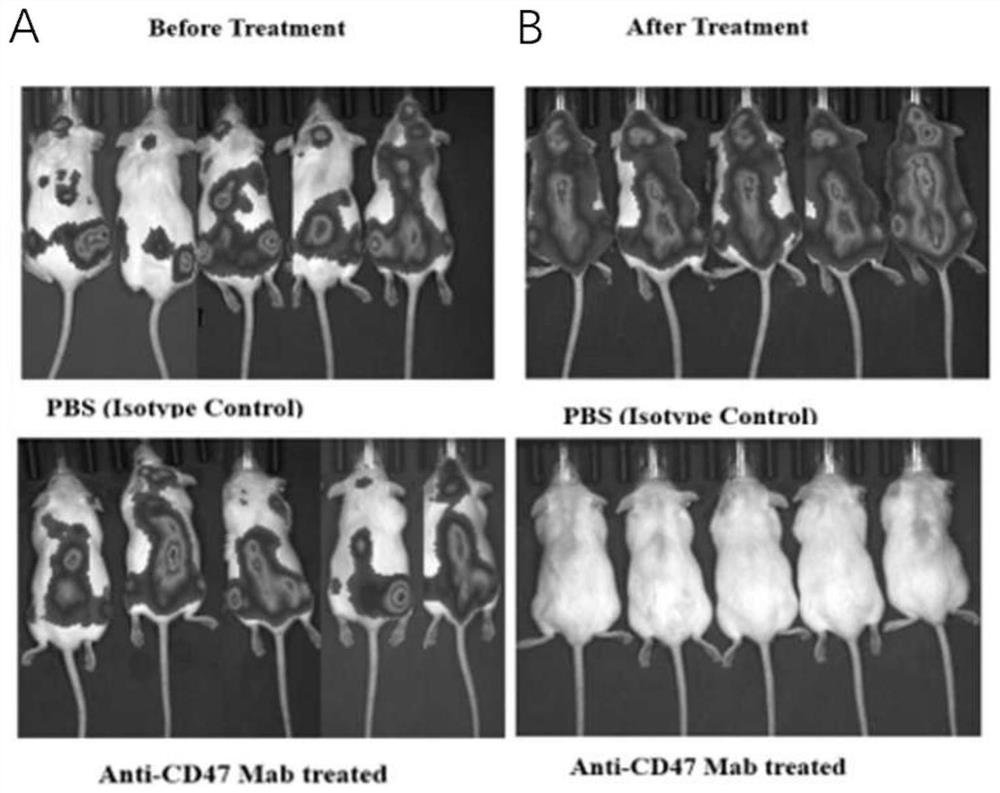
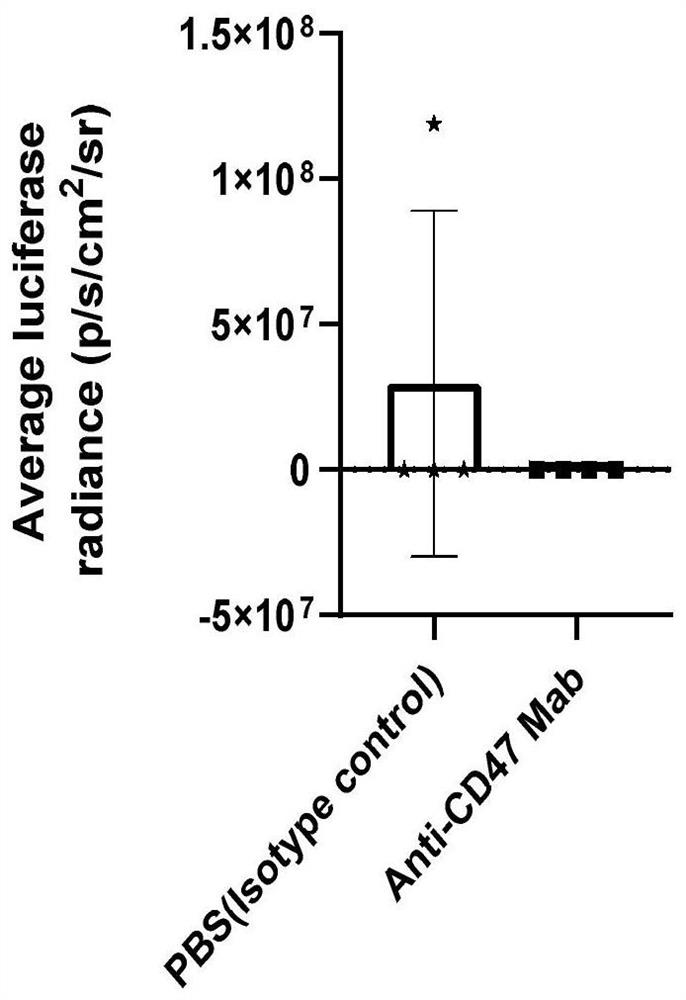
[0020] Example 3: In vivo effect of anti-CD47 monoclonal antibody against doxorubicin-resistant acute lymphoblastic leukemia subcutaneous xenograft tumor mouse model
[0021] NOD / SCID mice aged 3-4 weeks were selected, and the B-ALL drug-resistant mouse model with subcutaneous xenograft tumor was established with the doxorubicin-resistant acute lymphoblastic leukemia cell line Nalm6-Luc / ADR. Isolate the doxorubicin-resistant human B-ALL cell line Nalm6-Luc / ADR expressing luciferase in the logarithmic growth phase, counting 5×10 6 Cells were subcutaneously injected into the dorsal side of the right hind leg. After the successful construction of the subcutaneous transplanted tumor mouse model, they were divided into PBS negative control group and anti-CD47 monoclonal antibody treatment group, with 5 mice in each group, and the dosage of anti-CD47 monoclonal antibody was 100ug. per mouse, administered for 3 days, observed tumor growth, and recorded by IVIS bioluminescent imaging ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap