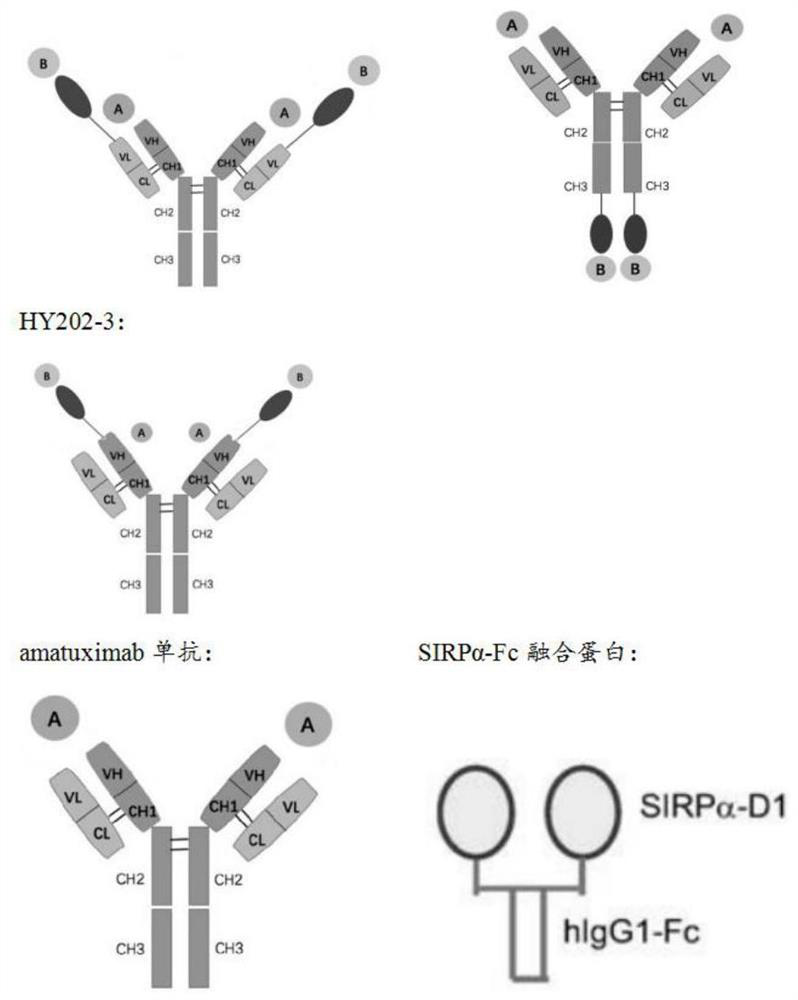
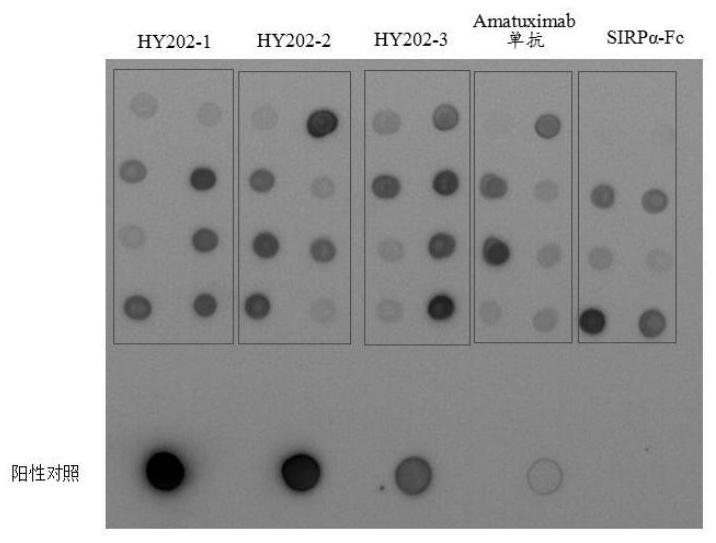
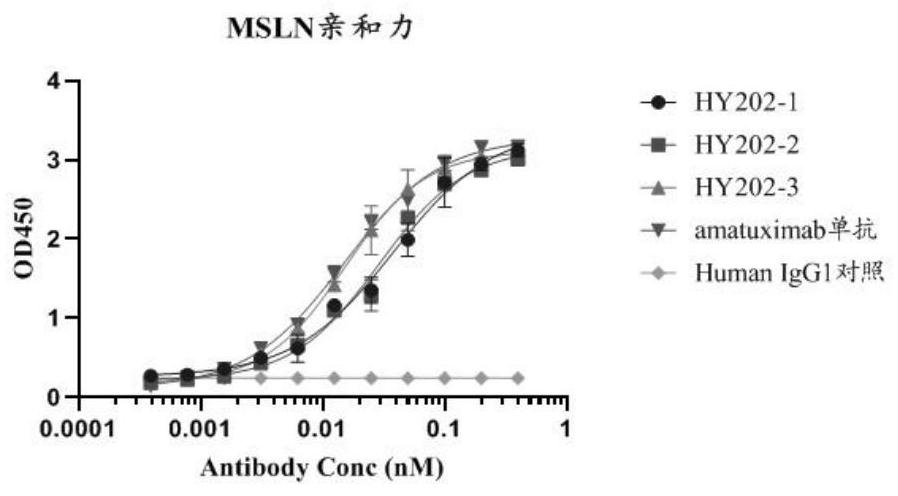
Recombinant bifunctional fusion protein and application
A fusion protein, bifunctional technology, applied in the field of fusion proteins, can solve the problem of lack of bispecific antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The preparation method of the fusion protein is not particularly limited in the present invention, preferably including artificially synthesizing the nucleotide sequences encoding the polypeptide chains of each fusion protein, and cloning them into vectors respectively, so as to construct HY202-1 and HY202-2 respectively and HY202-3 expression vector; use the expression vector to electroporate CHO-K1 cells, induce protein expression and purify. In the present invention, there are no special limitations on the methods of electroporation, protein expression induction and purification, and conventional technical means in the field can be used.
[0035] The present invention also provides the application of the above-mentioned fusion protein in the preparation of medicine for treating MSLN-positive tumors. The fusion protein of the present invention can combine with MSLN and CD47 at the same time, thereby exerting a therapeutic effect on MSLN-positive tumor cells through an...
Embodiment 1
[0039] 1. Expression vector construction
[0040] Antibody heavy chain using Amatuximab monoclonal antibody VH MSLN -CH1-Fc (SEQ ID NO.10), the gene sequence was sent to Nanjing GenScript Synthetics (project number: C9962EE050-2); the V SIRPαD1 (SEQ ID NO.11) was fused with the anti-human MSLN antibody light chain (SEQ ID NO.12), and the gene sequence was sent to Nanjing GenScript (Project No.: C9962EE050-4) to obtain the polypeptide chain V SIRPαD1 -VL MSLN -CL (SEQ ID NO. 13). The synthesized genes were respectively cloned into vectors to constitute HY202-1 expression vectors.
[0041] Anti-human MSLN antibody heavy chain VH MSLN -CH1-Fc (same as above) and V SIRPαD1 For gene fusion, the gene sequence was sent to Nanjing GenScript for synthesis (project number: C9962EE050-6) to obtain the polypeptide chain VH MSLN -CH1-Fc-V SIRPαD1 (SEQ IDNO.14); Antibody light chain adopts Amatuximab monoclonal antibody VL MSLN -CL (SEQ ID NO.15), the gene sequence was sent to Nanji...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com