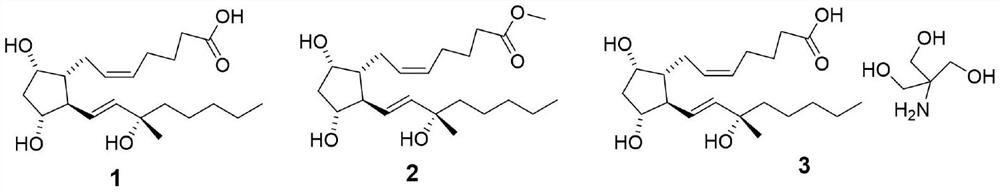
Carboprost 15-site isomer separation method
A technology of carboprost and isomers, which is applied in the field of impurity separation of carboprost 15-tertiary alcohol R isomers, can solve the problems of complex molecules, difficulties and uncertainties in industrialized large-scale production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 carries out example with protecting agent R=TBS
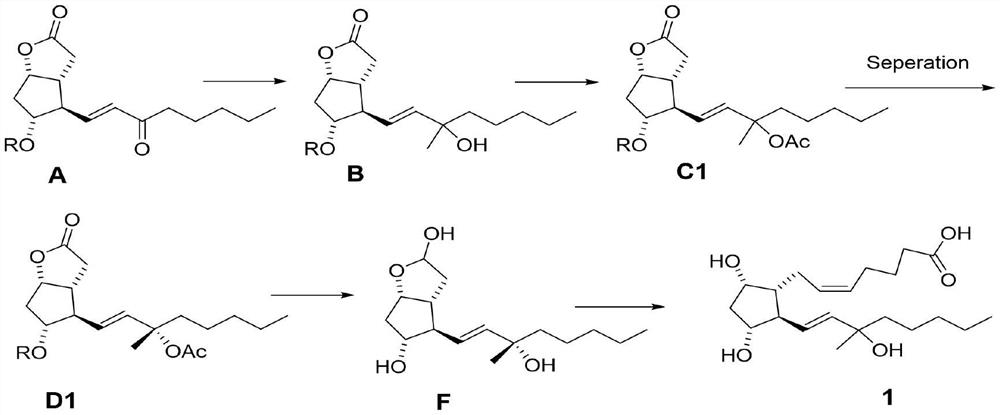
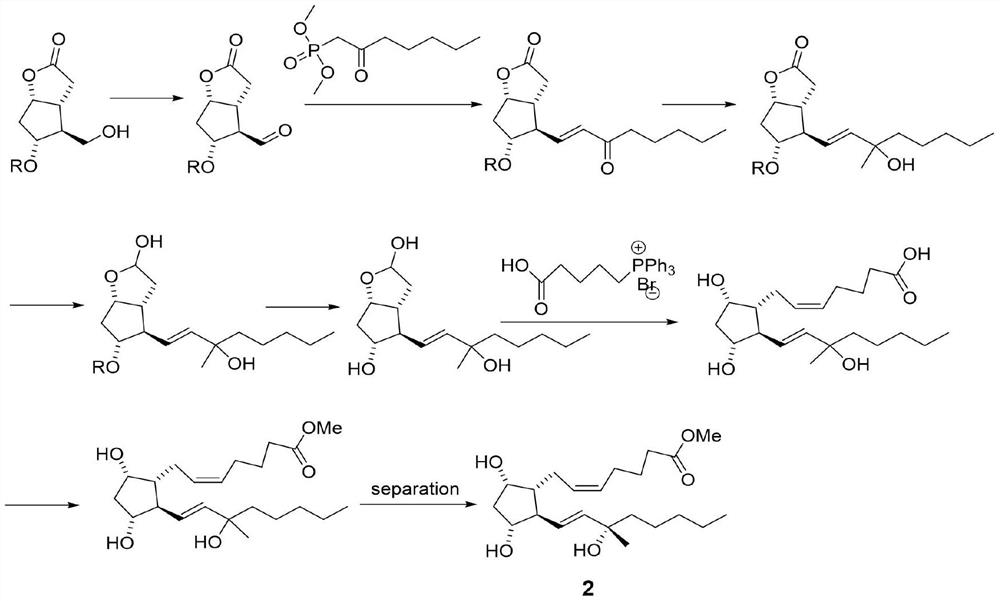
[0036] Step 1: Synthesize 60 g of compound A as a white solid according to the record in paragraph 0166-0178 of patent CN108602769A, with a yield of 95%.
[0037]Step 2: In a 2L three-necked flask, add compound A (55.0g, 145mmol), then add 800mL of toluene, under the protection of nitrogen, cool to below -50°C, start adding 3M MeMgCl tetrahydrofuran solution (120mL) dropwise, and detect the raw materials by TLC Disappeared completely, stopped responding. The temperature of the reaction solution was raised to 0° C., and 500 mL of a saturated ammonium chloride aqueous solution was slowly added dropwise thereto, and then a 3M aqueous hydrochloric acid solution was added dropwise until the pH=3-4. The organic layer was separated, and the aqueous layer was extracted with 200 mL of ethyl acetate. The organic phases were combined, washed successively with saturated aqueous sodium bicarbonate solution and saturated...
Embodiment 2
[0042] Embodiment 2 carries out example with protecting agent R=TES
[0043] Step 1: Synthesize 6.0 g of Compound A as a white solid according to the record in paragraph 0166-0178 of patent CN108602769A, with a yield of 95%.
[0044] Step 2: In a 2L three-necked flask, add compound A (5.5g, 14.5mmol), then add 80mL of toluene, under the protection of nitrogen, cool to below -50°C, start to drop 3M MeMgCl tetrahydrofuran solution (12mL), TLC detection The starting material disappeared completely and the reaction was stopped. The temperature of the reaction solution was raised to 0° C., and 50 mL of a saturated ammonium chloride aqueous solution was slowly added dropwise thereto, and then a 3M aqueous hydrochloric acid solution was added dropwise until the pH=3-4. The organic layer was separated, the aqueous layer was extracted with 20 mL of ethyl acetate, the organic phases were combined, washed successively with saturated aqueous sodium bicarbonate solution and saturated brin...
Embodiment 3
[0049] Embodiment 3 carries out example with protecting agent R=Bz
[0050] Step 1: Synthesize 30 g of compound A as an oily liquid according to the record in paragraph 0166-0178 of patent CN108602769A, with a yield of 80%.
[0051] Step 2: In a 1L three-neck flask, add compound A (25.0g), then add 400mL of toluene, under the protection of nitrogen, cool to below -50°C, start adding 3M MeMgCl tetrahydrofuran solution (60mL) dropwise, TLC detects that the raw material completely disappears , stop responding. The temperature of the reaction solution was raised to 0° C., and 250 mL of a saturated ammonium chloride aqueous solution was slowly added dropwise thereto, and then a 3M aqueous hydrochloric acid solution was added dropwise until the pH=3-4. The organic layer was separated, and the aqueous layer was extracted with 100 mL of ethyl acetate. The organic phases were combined, washed successively with saturated aqueous sodium bicarbonate solution and saturated brine, and drie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com