Methods for improving genome engineering and regeneration in plant ii
A technology for recombining genes and plant cells, applied in botany equipment and methods, biochemical equipment and methods, plant regeneration, etc., can solve problems that hinder the routine implementation of transient gene editing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0388] Example 1. Transient co-expression of a booster gene and a gene of interest (GOI) by co-bombardment.
[0389] Gene cloning and construct preparation
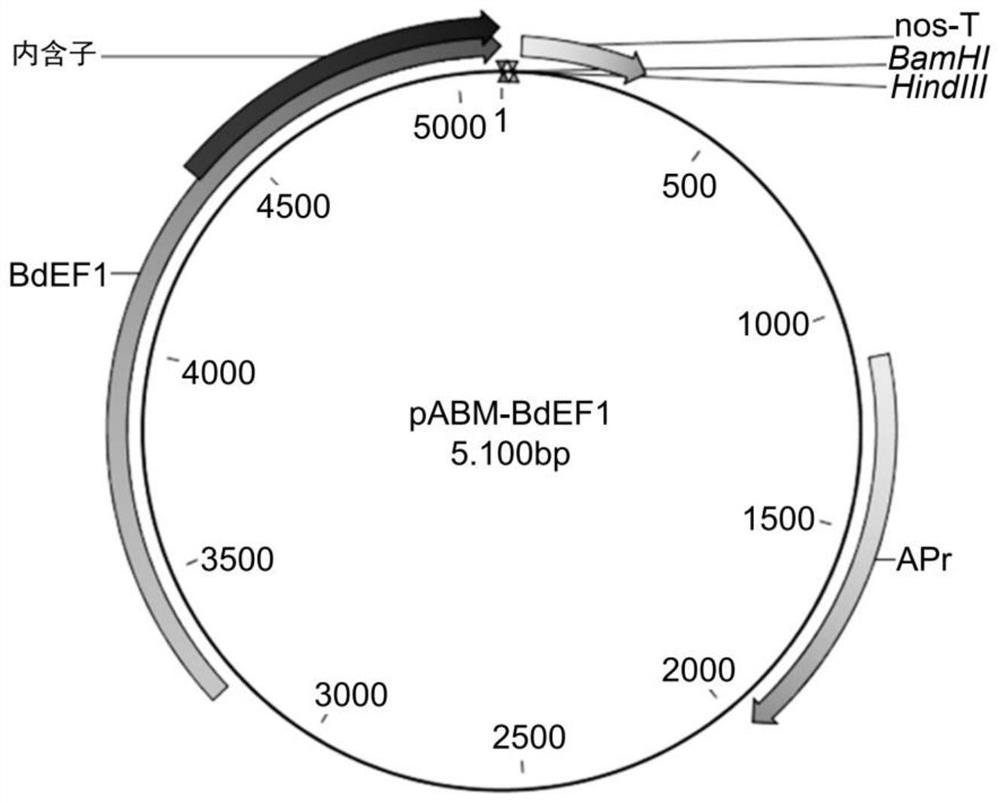
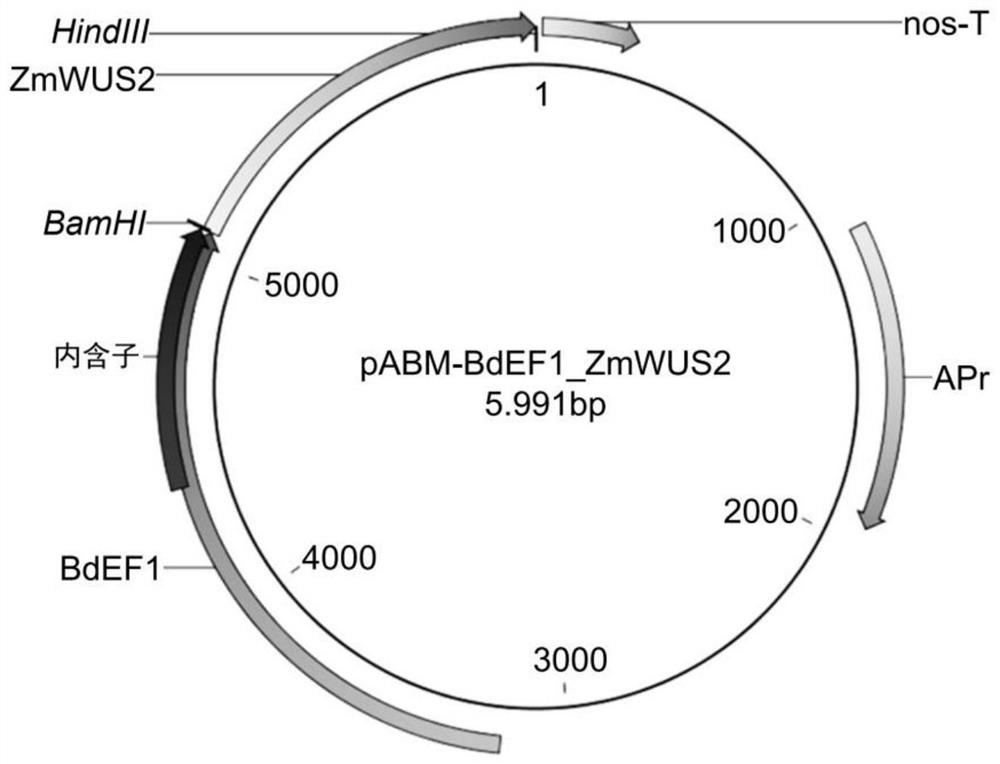
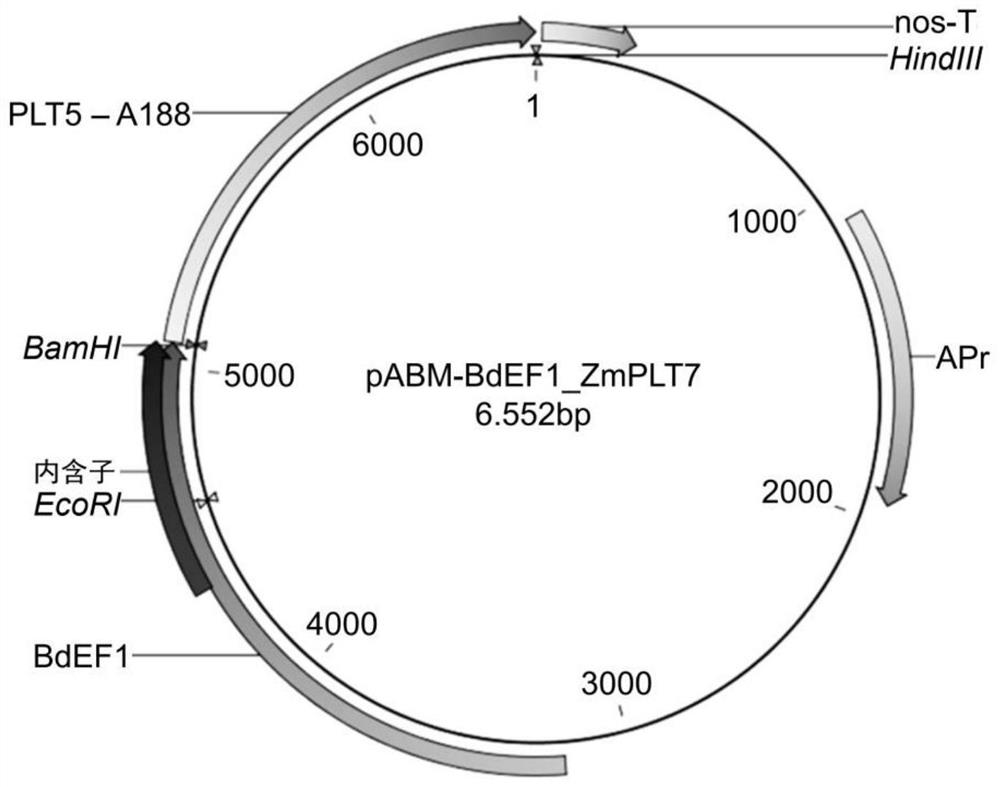
[0390] Maize WUS2 (ZmWUS2) and PLT7 (ZmPLT7) genes were cloned by RT-PCR using total RNA isolated from maize immature embryos of genotype A188. Wheat RKD4 and RKD2 and KWS-RBP1 genes were maize codon-optimized from their protein sequences and synthesized by Integrated DNA Technologies (IDT, San Diego, CA, USA). The enhanced gene fragment was cloned into the expression vector pABM-BdEF1 at the cloning sites of BamHI and HindIII ( figure 1 ) and expressed under the control of the BdEF1 promoter (pBdFE1) and the nos terminator (nos-T). pBdFE1 is a strong constitutive promoter from Brachypodium. Maps of the sequence-confirmed constructs are shown in Figures 2 to 5 and 8 in.
[0391] Maize immature embryos prepared for bombardment
[0392] 9-12 days after pollination, ears of corn (ie A188 or Hi II) with immature em...
Embodiment 2
[0400] Example 2. Transient coexpression of a combination of ZmWUS2 and ZmPLT7 in maize A188 immature embryos promotes early embryogenesis and regeneration.
[0401] Transient co-delivery, embryo preparation and culture are described in Example 1 above. For each bombardment, four premixed DNA plasmids were coated on 100 μg of gold particles with a size of 0.4 μm and co-introduced into the embryonic cotyledon cells of A188 immature embryos at a burst pressure of 650 psi. For one bombardment, the four plasmids were premixed as follows:
[0402] -100ng enhances ZmPLT5 or ZmPLT7 ( figure 2 with image 3 )
[0403] -200ng KWS-RBP1( Figure 4 )
[0404] -100ng pGEP359( Image 6 )
[0405] -150ng pGEP324( Figure 7 )
[0406] Embryos with dense fluorescent signals under a fluorescence microscope were selected ( Figure 9 ), and transferred it from N6OSM to N6-5Ag to induce embryogenic callus. Selected embryos were cultured on N6-5Ag plates with the embryos cotyledons faci...
Embodiment 3
[0411] Example 3. Transient Expression of ZmPLT7 in Maize Hi Immature Embryos Improves Stable Transformation of Co-delivered Reporter Genes
[0412] Examples 1 and 2 describe the preparation of maize embryos, transient bombardment and induction of embryogenic callus. Embryos were cultured in N6-5Ag medium at 27°C in the dark for 14 days. tDT fluorescence was used to monitor the induction and stable transformation of embryogenic callus by observation under a fluorescence microscope. Specifically, boosting was measured by the ability of the absence of selection to increase the transformation frequency (TF) of the tDT reporter gene 12 days after bombardment.
[0413] from Figure 14 The strong and uniform tDT fluorescence signal of the newly generated embryogenic structures indicates the integration and stable transformation of the tDT gene. Stable transformation frequency was defined as the number of embryos induced with at least one stable tDT fluorescent construct from the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| breaking strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com