Anti-PD-L1 nano antibody and application thereof
A technology of PD-L1 and nano-antibody, which is applied in the field of biomedicine, can solve the problem that the affinity has not reached the ideal state, and achieve the effect of high affinity, weak immunogenicity and significant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: Alpaca immunization and construction of phage immune library
[0062] Alpaca immunization: immunize a healthy alpaca (vicugna pacos, alpaca, lama pacos) with PD-L1 extracellular region recombinant protein (Shenzhou, 10084-H08H), 0.11mg protein per immunization, a total of 5 times, the first time Freund's complete adjuvant CFA was used, and Freund's incomplete adjuvant IFA was used for the second to fifth times to emulsify and mix with the antigen, and then subcutaneously inject multiple points.
[0063] Building a library: Take 50ml of peripheral blood to separate peripheral blood mononuclear cells (PBMC), extract PBMC total RNA, reverse transcribe into cDNA by RT-PCR, design primers for PCR amplification of VHH genes, and build an immune library. Peripheral blood lymphocytes were separated, and 50 mL of peripheral blood was collected. PBMCs were separated according to the instructions of the lymphocyte separation medium, and total RNA of PBMCs was extracted ...
Embodiment 2
[0064] Example 2: Screening and Identification of Nanobody Immune Library
[0065] Two rounds of screening were performed with the recombinant protein in the extracellular region of PDL1.
[0066] The first round of panning: PDL1-hFc (Protein No. QP004) and hFc protein were respectively coated in immunotubes, 50 nM, 1 ml, overnight at 4°C. Blocking: Block the immunotube with 2% Milk / PBS at 37°C for 1 hour. Subtraction: Pour off the blocking solution, add 900 μl 2% Milk / PBS to the hFc-coated immunotube, then add 100ul phage immune library, and rotate at room temperature for 1 hour. Binding: transfer the phage immune library to the immune tube coated with PDL1-hFc, and rotate at room temperature for 1 hour. Washing: Wash the immunotube 5 times with 1×PBST, and 5 times with 1×PBS. Elution: 800 μl 100 mM TEA, 10 min at room temperature. Neutralization: Transfer the eluate to a 1.5ml EP tube and add 400μl 1M pH 7.4 Tris. Infection: The eluted phages after neutralization were a...
Embodiment 3
[0077] Example 3: Nanobody construction of FC fusion protein, cloning, expression, and purification of protein
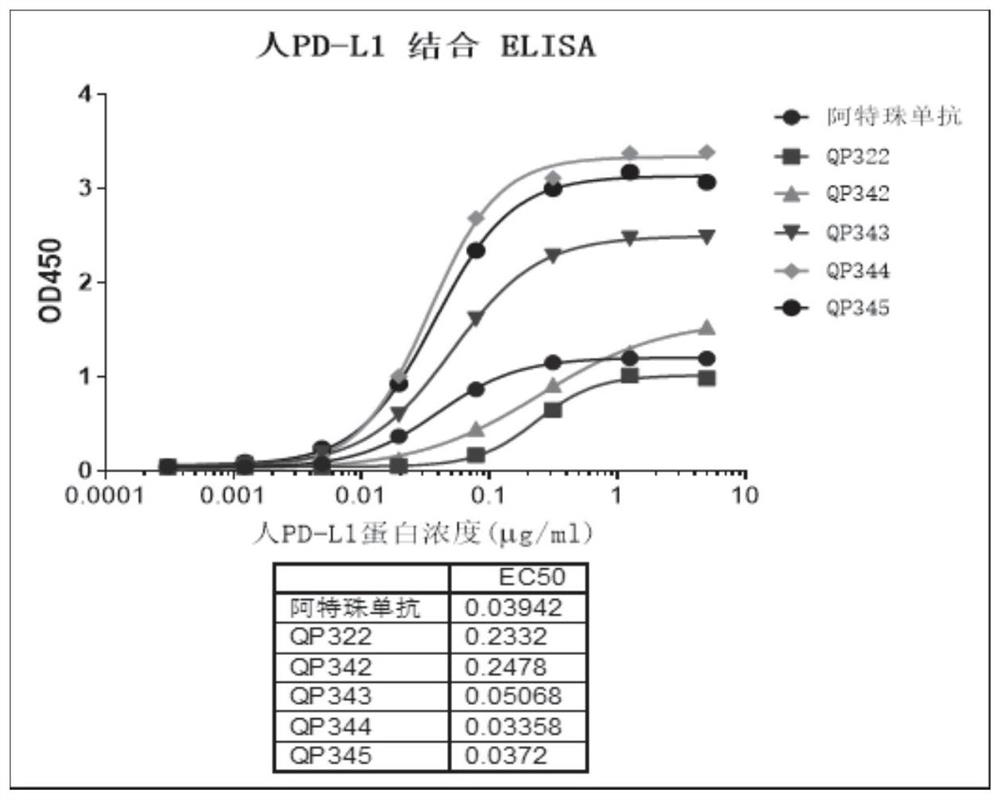
[0078] Cloning design and construction: 20 clones were transformed into PD-L1-FC fusion protein whose C-terminus is human IgG1 FC. The reconstituted plasmid was expressed in HEK293 cells, purified by protein A affinity chromatography, and a total of 20 candidate PD-L1 VHH-FC fusion proteins QP326-QP345 were obtained, the sequences of which were shown in SEQ ID NO: 1-20. The rear end of the antibody is connected to the human IgG1 Fc segment, such as the sequence SEQ ID NO: 61. The numbering of the Fc fusion protein of the corresponding Nanobody is formed by adding the suffix Fc to the corresponding Nanobody.
[0079] In addition, QP322 is used as a reference in the examples, and the patent CN201910567277.5 can be referred to, and its anti-PD-L1 VHH sequence is:
[0080] EVQLLESGGGLVQPGGSLRLSCAASGFTYGTYAMSWFRQAPGKGREGVACIDIYGRASYTDPVKGRFTISQDNSKNTLYLQMNSLKAEDTAVYYCA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com