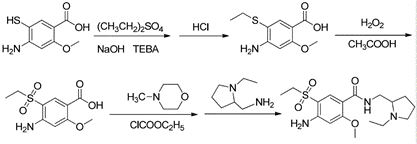
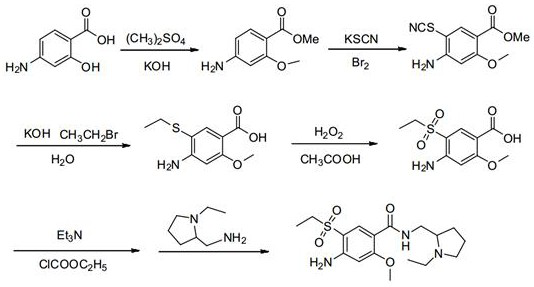
Preparation method of important intermediate of amisulpride
A synthetic method and amino technology, applied in mercaptan preparation, organic chemistry, etc., can solve the problems that the characterization of intermediates and the synthesis process have not been studied in depth, and the impact of quality and yield has not been well understood and analyzed. , to achieve the effect of simple and practical synthesis method, stable yield and improved yield purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
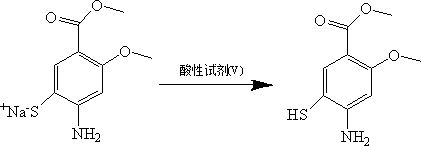
[0020] 0.5 g of methyl 4-amino-2-methoxy-5-thiocyanatobenzoate was dissolved in 6 ml of water and 3 ml of methanol while maintaining the temperature of the solution at 10-15°C and stirring. 0.26g Na 2 S was dissolved in 2 ml of water and added dropwise to the solution. After stirring for 1 h, the reaction temperature was raised to 80°C, and the reaction was continued for 2 h. The reaction mother liquor was filtered, and the pH value of the filtrate was adjusted to 2-3 using 3M hydrochloric acid, and 0.38 g of a yellow solid was obtained by filtration, with a yield of 85%.
Embodiment 2
[0022] 0.5 g of methyl 4-amino-2-methoxy-5-thiocyanatobenzoate was dissolved in 6 ml of water and 3 ml of ethanol, while maintaining the temperature of the solution at 5-10° C. and stirring. 0.26g Na 2 S was dissolved in 2 ml of water and added dropwise to the solution. After stirring for 1 h, the reaction temperature was raised to room temperature, and the reaction was continued for 8 h. The reaction mother liquor was filtered, and the pH value of the filtrate was adjusted to 5-6 with 3M acetic acid, and 0.35 g of a yellow solid was obtained by filtration, with a yield of 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com