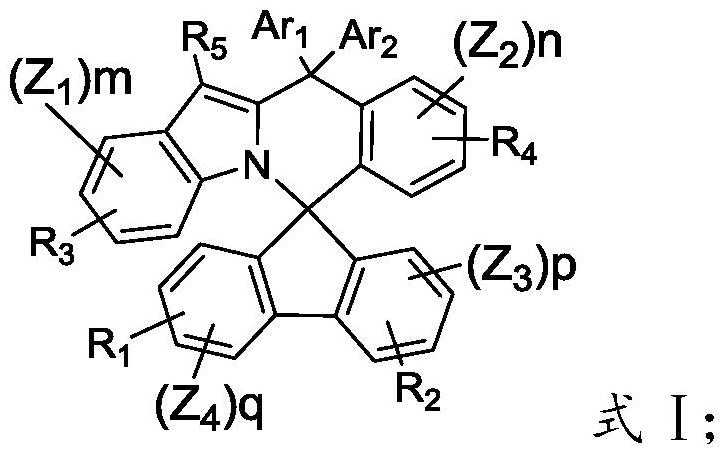
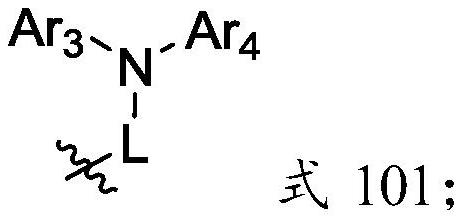
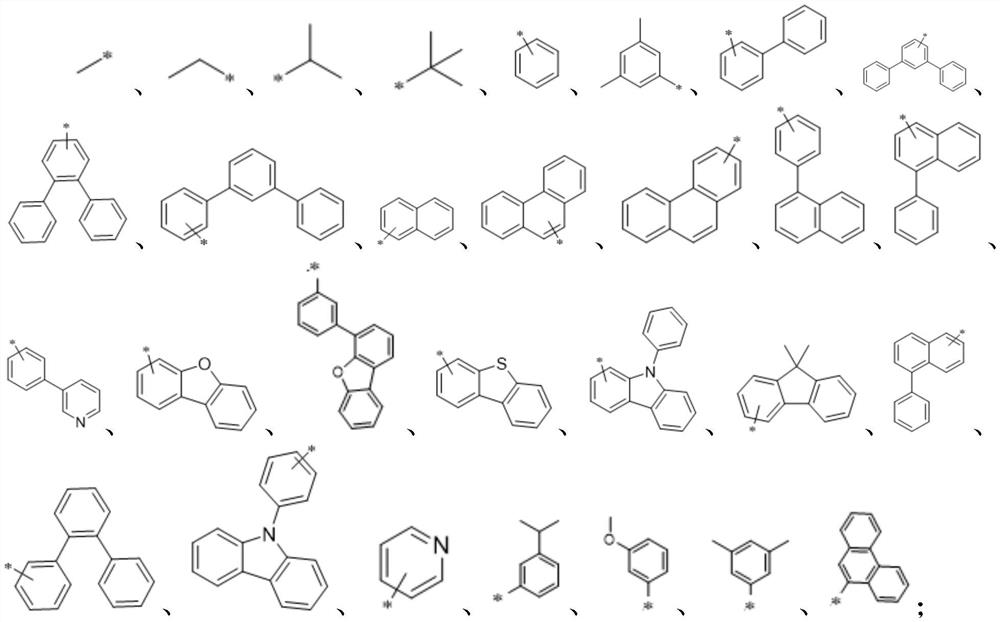
A kind of nitrogen-containing heterocyclic organic electroluminescent compound and its preparation method and application
A technology of luminescence and compounds, which is applied in the field of organic electroluminescence compounds and their preparation, can solve problems such as low lifespan, long time-consuming, complex synthesis process, etc., and achieve high photothermal stability, low ionization potential, high hole The effect of mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] The present invention provides a method for preparing an organic electroluminescent material containing a nitrogen heterocycle described in the technical solution, comprising the following steps:
[0057] Combine intermediate 4 with (HO) 2 -B-L-Hal 3 Reaction, obtain intermediate 5;
[0058] The intermediate 5 and Ar 3 -NH-Ar 4 The materials are reacted in a solvent to obtain an organic electroluminescent material having a structure of formula I;
[0059]
[0060] If the L is not a connecting key, then the Hal 1 、Hal 2 and Hal 3 independently selected from bromine or iodine;
[0061] If the L is a connecting bond, the intermediate 4 is directly connected to Ar 3 -NH-Ar 4 The material reacts, and the Hal 1 、Hal 2 and Hal 3 independently selected from chlorine.
[0062] In the present invention, the intermediate 4 is preferably prepared according to the following method:
[0063] React intermediate 3 with glacial acetic acid under heating conditions to ob...
Embodiment 1
[0088]
[0089] 1. Under the protection of nitrogen, add raw material A (9.80g, 50.00mmol) into a three-necked flask, add 100.00ml of anhydrous tetrahydrofuran, then cool the reaction system to -78°C, add dropwise n-BuLi (3.20g, 50.00 mmol), stirred at -78°C for 2h. Material B (3.48g, 60.00mmol) was dissolved in 35.00ml of tetrahydrofuran solution, and then added dropwise to the above reaction system. After the dropwise addition, the temperature was raised to room temperature, and stirred for 10h. Then, a saturated ammonium chloride solution was added to quench the reaction, the reaction solution was extracted three times with ethyl acetate, the organic phases were combined, washed with water and saturated brine, and dried over anhydrous magnesium sulfate. Then the dried solid was added to the ethanol solution, and the temperature was raised to 80 ° C, stirred for 5 hours, and then the solution was filtered while hot to obtain a solid, which was then rinsed with petroleum e...
Embodiment 2
[0105]
[0106] 1. Under the protection of nitrogen, add raw material A-73 (9.80g, 50.00mmol) into a three-necked flask, add 90.00ml of anhydrous tetrahydrofuran, then cool the reaction system to -78°C, add dropwise n-BuLi (3.20g , 50.00mmol), stirred at -78°C for 2h. The raw material B-73 (10.93g, 60.00mmol) was dissolved in 100.00ml of tetrahydrofuran solution, and then added dropwise to the above reaction system. After the dropwise addition, the temperature was raised to room temperature, and stirred for 10h. Then, a saturated ammonium chloride solution was added to quench the reaction, the reaction solution was extracted three times with ethyl acetate, the organic phases were combined, washed with water and saturated brine, and dried over anhydrous magnesium sulfate. Then the dried solid was added to the ethanol solution, and the temperature was raised to 80 ° C, stirred for 5 hours, and then the solution was filtered while hot to obtain a solid, which was then rinsed w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com