Application of butenyl phthalide and method for preparing butenyl phthalide into pharmaceutical composition
A technology of butenylphthalide and butenylbenzene, which is applied to the application of butenylphthalide and its preparation as a pharmaceutical composition, can solve problems such as threats to human health, and achieve the treatment or prevention of neurodegenerative diseases , the effect of improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Synthetic Hydrazine Butylphthalide
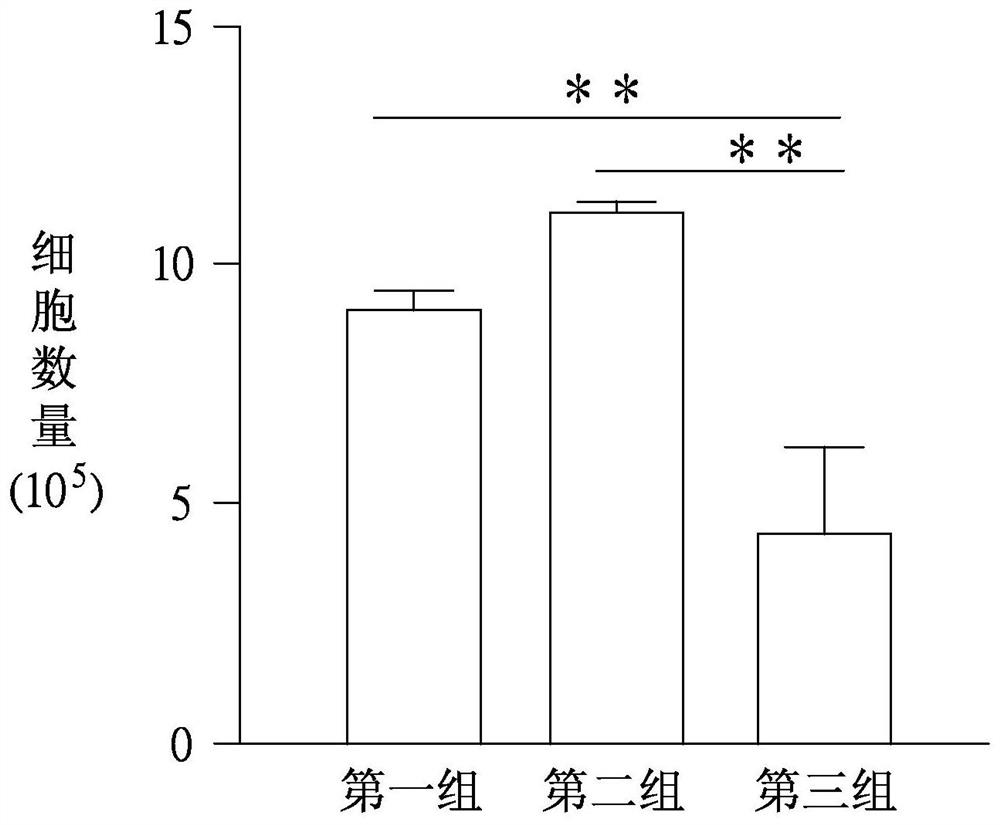
[0041] Butenyldenephthalide, BDPH, W333301) and F127 polymer substance were obtained from Sigma-Aldrich, US Sigma-Aldrich (PLURONIC F127, P2443). After mixing 10 mg-butardylphenylphthalide and F127 polymer substances in a ratio of 1: 1 or 1: 2, it was dissolved in Tetrahydrofuran, and then added to 10 ml of water, and then heated quickly Tetrahydrofuran was removed to perform freeze drying. Thereafter, the dissolved in water is rehydrated, and the olymiacphphiphenylene particles after the emulsion in the solution are about 30 to 200 nm, and the polydispersity index is 0.2-0.5.
Embodiment 2
[0042] Example 2: Cell culture
[0043] Culture human universal stem cells in serum-free medium Essential 8 TM (Life Technology, United States), and Matrigel TM Bastropin (Becton-Dickinson, USA) is attached. Unexplified the culture fluid, washed with phosphate buffer 2 times, add an enzyme ACCUTASE TM (Merck Millipore, the United States) reacted 2 to 5 minutes later in culture liquid, and the cells were scattered into a small group, and centrifuged 1000 rpm, 2 minutes, and then absorbed the supernatant, and put it in new culture. The culture was cultured for about 3 to 5 days, and the culture fluid was replaced daily during culture during culture.
Embodiment 3
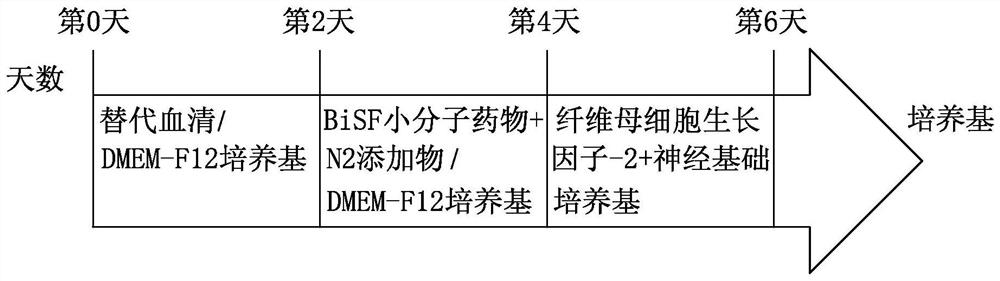
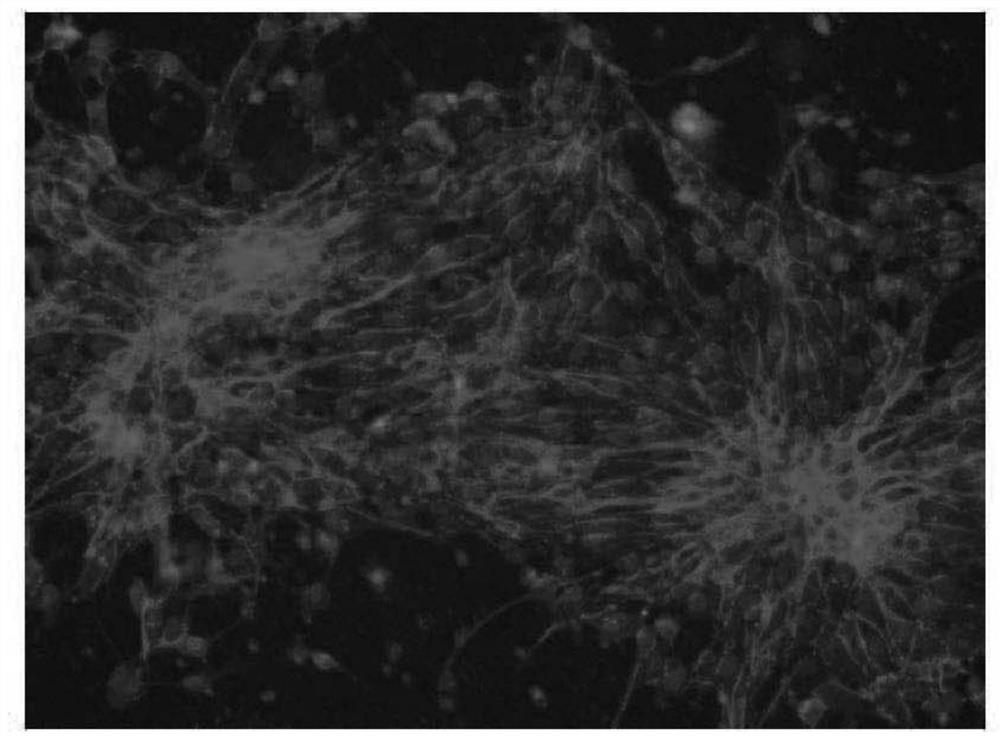
[0044] Example 3: Neuronal differentiation
[0045] Human universal stem cells were cultured to 8-9, and the cells were rinsed with phosphate buffer 2 times, with an enzyme ACCUTASE TM After 2 ~ 5 minutes, the culture solution was added to the enzyme, then rinsed up the cells, scattered until it was appropriate for about 2 minutes, and then placed in the cells in a 20% replacement serum (Knock Out SerumReplacement). DMEM-F12 medium referred to as KSR, Life Technology, USA), was suspended for 2 days, and the Embryonic body suspension was obtained.
[0046] The suspension type embryoid is for the centrifuge tube. After natural settlement, the supernatant is removed, and the neurological inducing medium containing BISF small molecular drug is submitted for 2 days, then the suspended type embryo has a cyclic epithelial cell. Structure, where small molecule drugs contain 0.5 μm BiO (Sigma-Aldrich, USA), 10 ng / ml of fibroblast growth factor-2 (FGF-2, Peprotech, USA) and 10μm SB431542 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com