Preparation of bimetallic catalyst and its application in catalytic depolymerization of lignin C-C bond
A bimetallic catalyst, lignin technology, applied in catalyst activation/preparation, preparation of organic compounds, molecular sieve catalysts, etc., can solve the problems of single product selectivity to be improved, low aromatic compound selectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
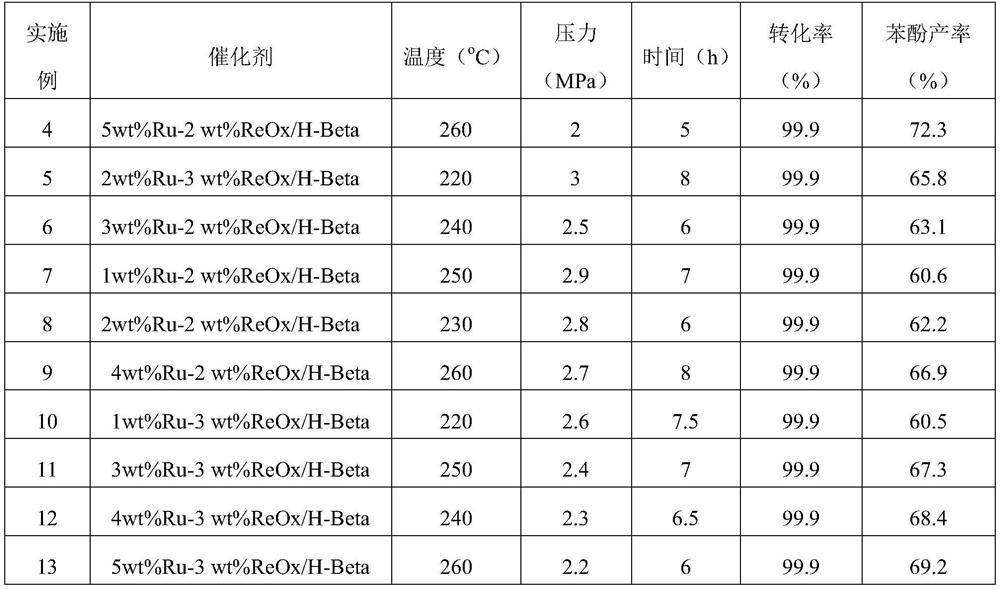
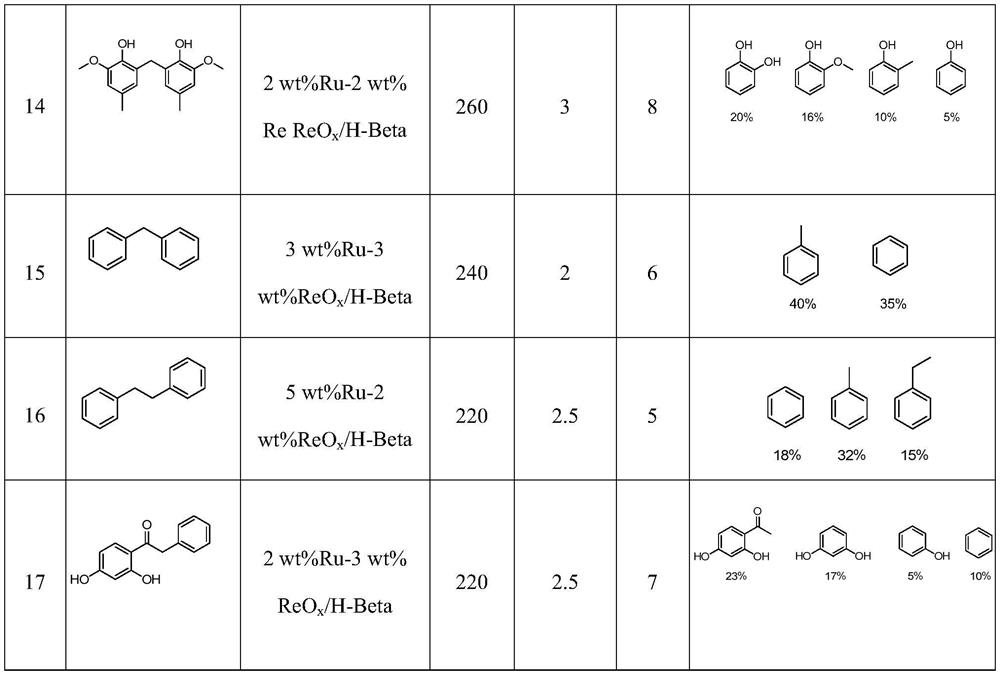
Examples
Embodiment 1
[0036] Bimetallic Catalyst Ru-ReO x Preparation of / H-Beta (2wt%Ru, 1wt%Re):
[0037] (1) Dry H-Beta at a high temperature of 500°C for 3 hours;
[0038] (2) Dissolve 30.4 mg of ammonium perrhenate in ultrapure water to obtain 0.8 g of ammonium perrhenate solution, place the dried 1 g of H-Beta in the ammonium perrhenate solution, and stir evenly to obtain a solid-liquid mixture A -1;
[0039] (3) The solid-liquid mixture A-1 was left to stand at 25°C for 5 hours, then dried at 60°C for 8 hours, and finally dried at 110°C for 8 hours, and ground to obtain solid A'-1;
[0040] (4) 52.6mg RuCl 3 ·3H 2 O was dissolved in 747 mg ultrapure water to give 0.8 g RuCl 3 solution, the solid A'-1 obtained in (3) was placed in RuCl 3 In the solution, stir evenly to obtain a solid-liquid mixture B-1;
[0041] (5) The solid-liquid mixture B-1 was left to stand at 25°C for 5 hours, then dried at 60°C for 8 hours, and finally dried at 120°C for 8 hours to obtain solid B'-1;
[0042] (...
Embodiment 2
[0044] Bimetallic Catalyst Ru-ReO x Preparation of / H-Beta, x=4-7 (3wt%Ru, 3wt%Re):
[0045] (1) Dry H-Beta at a high temperature of 600°C for 5 hours;
[0046] (2) Dissolve 45.6 mg of ammonium perrhenate in ultrapure water to obtain 0.8 g of ammonium perrhenate solution, place the dried 1 g of H-Beta in the ammonium perrhenate solution, and stir evenly to obtain a solid-liquid mixture A -2;
[0047] (3) The solid-liquid mixture A-2 was placed at 23°C for 5.5 hours, then dried at 70°C for 8 hours, and finally dried at 120°C for 8 hours, and ground to obtain solid A'-2;
[0048] (4) 79.5mg RuCl 3 ·3H 2 O was dissolved in 720.5 mg ultrapure water to give 0.8 g RuCl 3 solution, the solid A'-2 obtained in (3) was placed in RuCl 3 solution, stir evenly to obtain solid-liquid mixture B-2;
[0049] (5) Dry the solid-liquid mixture B-2 at 23°C for 5.5h, then at 70°C for 9h, and finally at 120°C for 9h to obtain solid B'-2;
[0050] (6) Grind the solid B'-2 evenly, place it in ...
Embodiment 3
[0052] Bimetallic Catalyst Ru-ReO x Preparation of / H-Beta, x=4-7 (5wt%Ru, 2wt%Re):
[0053] (1) Dry H-Beta at a high temperature of 550°C for 4 hours;
[0054] (2) Dissolve 30.4 mg of ammonium perrhenate in ultrapure water to obtain 0.8 g of ammonium perrhenate solution, place the dried 1 g of H-Beta in the ammonium perrhenate solution, and stir evenly to obtain a solid-liquid mixture A -3;
[0055] (3) Place the solid-liquid mixture A-3 at 20°C for 6h, then dry at 80°C for 10h, and finally dry at 120°C for 10h, and grind to obtain solid A'-3;
[0056] (4) 131.5mg RuCl 3 ·3H 2 O was dissolved in 668.5 ultrapure water to obtain 0.8 g RuCl 3 solution, the solid A'-3 obtained in (3) was placed in RuCl 3 solution, stir evenly to obtain solid-liquid mixture B-3;
[0057] (5) Stand the solid-liquid mixture B-3 obtained in (4) at 20°C for 6h, then dry at 80°C for 10h, and finally dry at 120°C for 10h to obtain solid B'-3;
[0058] (6) Grind the solid B'-3 evenly, place it in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com