Application of EGFR/HER2 receptor tyrosine kinase inhibitor in preparation of medicine for treating HER2 mutation cancer
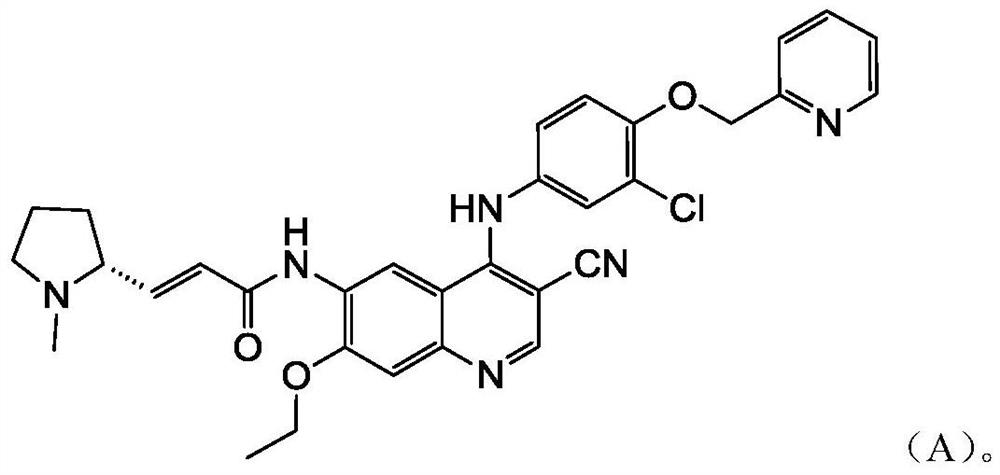
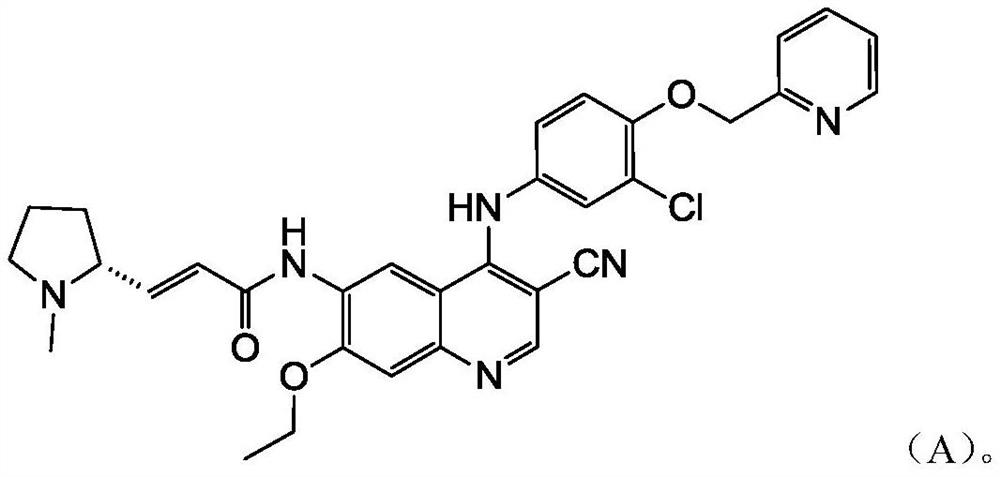
A technology of inhibitors and uses, applied in the field of application of EGFR/HER2 receptor tyrosine kinase inhibitors in the preparation of drugs for the treatment of HER2 mutation cancer, capable of solving problems such as the effect of compounds of formula A not disclosed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
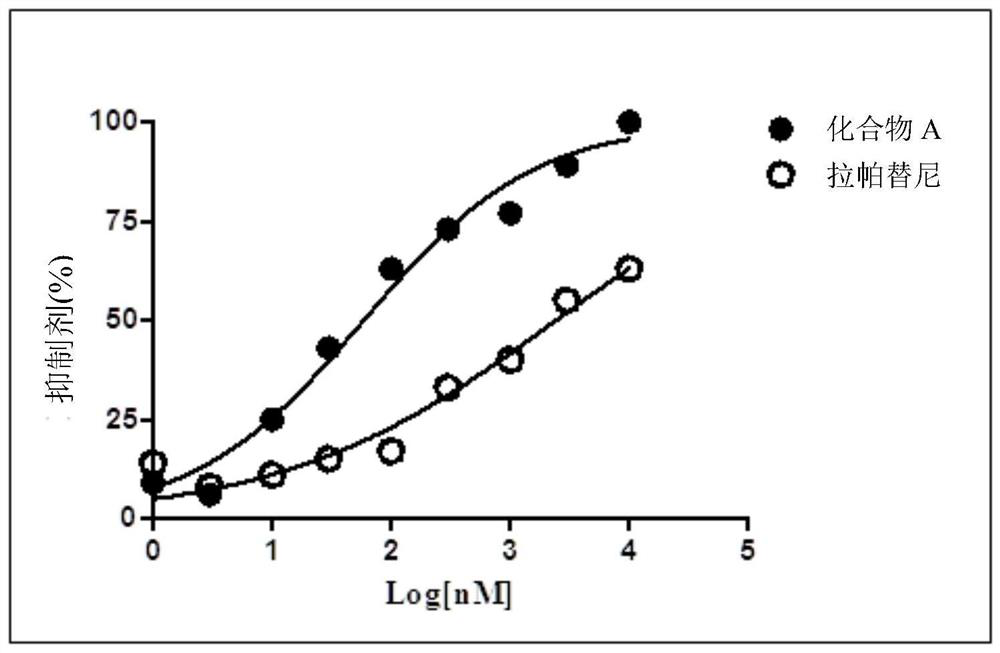
[0021] Example 1: Effects of compound A and lapatinib on the proliferation of ATCC H1781 cells cultured in vitro.
[0022] 1. Test drugs
[0023] Drug name: compound A dimaleate (batch number S0915100514), lapatinib di-p-toluenesulfonate (batch number 20090105). Preparation method: all prepared with DMSO.
[0024] 2. Cell lines
[0025] ATCC H1781 cells come from Shanghai Lung Hospital, the cells are HER2 mutation (InsG776V, C), the patient is a 66-year-old female Caucasian lung adenocarcinoma patient, cultured with PRIM 1640 medium containing 10% fetal bovine serum (FBS).
[0026] 3. Reagents and instruments
[0027] PRIM 1640 was purchased from Gibco BRL; fetal bovine serum was purchased from Gibco; multifunctional microplate reader was purchased from BioTek; sulforhodamine B (SRB) was purchased from Sigma.
[0028] 4. Test method
[0029] SRB protein staining was used to detect the inhibitory effect of drugs on the proliferation and growth of tumor cells. The main ste...
Embodiment 2
[0038] Example 2: Effect of compound A and lapatinib on the activity of HER2 recombinant protease in vitro
[0039] 1. Test drugs
[0040] Compound A (batch number SHR120201-002-06), and lapatinib (batch number SHR115758-010-17) were provided by Jiangsu Hengrui Medicine, and staurosporine was purchased from MedChem (Monmouth Junction, NJ) in the United States (batch number MC -2104).
[0041] 2. Recombinant protein
[0042] Recombinant human protein HER2 WT (Lot#W353-1) and five HER2 mutant proteins (A775_G776insYVMA: lot#Z1251-6; D769H: lot#K1683-5; D769Y: lot#P1688-9; V777_G778insCG: lot#Z1287-3 ; V777L: lot#K1850-3) were purchased from SignalChem (Richmond, BC V6V 2J2, CANADA). These recombinant proteins are polypeptides from amino acid 676 of HER2 protein to amino acid 1255 at the C-terminus, all of which were produced by baculovirus in Sf9 insects Expressed in cells, and the N-terminus is tagged with GST. The EGFR gene index number is NM_004448. The protein purity of...
Embodiment 3
[0049] Example 3: Effect of compound A and lapatinib on proliferation of HER2 mutant MCF10A cell line cultured in vitro.
[0050] 1. Test drugs
[0051] Drug name: compound A (lot number S0915151219), lapatinib (lot number SHR115758-010-17). Preparation method: all prepared with DMSO.
[0052] 2. Cell lines
[0053] MCF10A cells were purchased from ATCC, and the cells were used as mother cells. The vector GV341 was used to package overexpressed lentivirus, and then infected with lentivirus to establish 19 mixed clone stable cell lines, including empty vector control (NC), HER2 WT, HER2YVMAdup, P780_Y781insGSP , G776>VC, V777L, L755S, D769H, G776R, G776C, L755P, V842I, L866M, R896C, S310F, S310Y, G309A, G309E, and D769Y. All cells were cultured with DMEM / F12 medium plus 5% horse serum, 20ng / ml EGF, 10μg / ml insulin, 0.5μg / ml hydrocortisone, 1% penicillin / streptomycin (P / S) and 100ng / ml Cholera Toxin.
[0054] 3. Reagents and instruments
[0055] DMEM / F12 (Gibco, 10-092-CVR)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com