Amide compound and medical application thereof as STING inhibitor
A technology of amide compounds and solvates, which is applied in the field of biomedicine and can solve problems such as weak activity and large side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
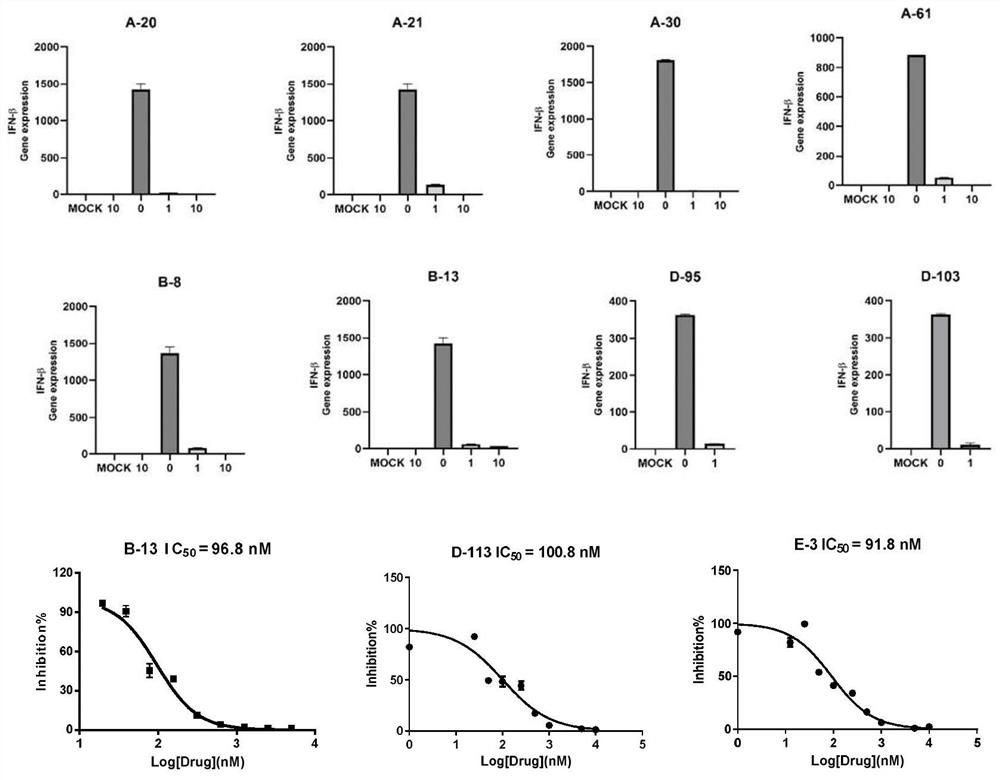
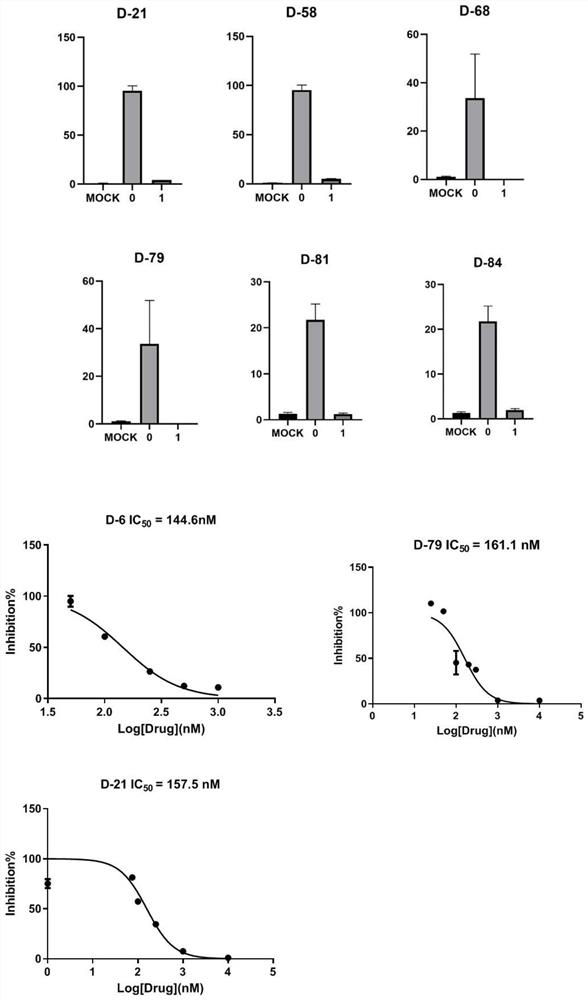
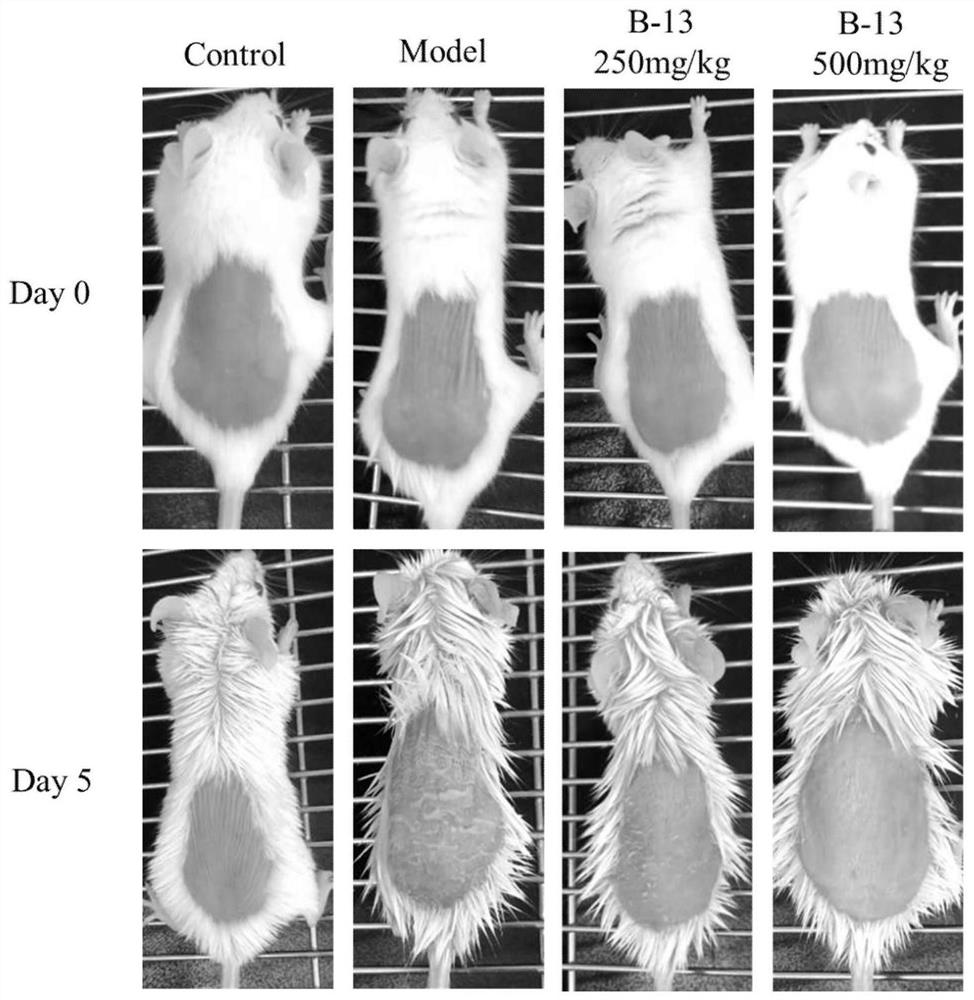
Image
Examples
Embodiment 1
[0127] N-(3-((4-fluorophenyl)sulfonylamino)-4-hydroxyphenyl)-3'-sulfamoyl[1,1'-biphenyl]-4-carboxamide (Compound A-1 )
[0128]
[0129]
[0130] A THF (2 mL) solution of compound I-0 (3-bromobenzenesulfonyl chloride) (610 mg, 2.39 mmol) was added dropwise into aqueous ammonia (6 mL), and reacted at room temperature for 5 h. The reaction solution was extracted with EtOAc (20mL), and the organic phase was washed with water (10mL) and saturated brine (10mL) successively, and anhydrous Na 2 SO 4 After drying, filtering, and evaporating the solvent under reduced pressure, the compound I-1 (534 mg, 95%) was obtained as a white solid.
[0131] Compound I-1 (238mg, 1.01mmol), p-methoxycarbonylphenylboronic acid (200mg, 1.11mmol), anhydrous K 2 CO 3 (279mg, 2.02mmol), Pd(PPh 3 ) 4 (58 mg, 0.05 mmol) was added to Toluene (Tol) (10 mL) and MeOH (2 mL), and reacted for 3 h under nitrogen atmosphere at 80°C. Cool at room temperature, filter and wash the filter cake with EtOAc...
Embodiment 2
[0138] N-(3-((4-fluorophenyl)sulfonylamino)-4-hydroxyphenyl)-4'-methoxy-3'-sulfamoyl[1,1'-biphenyl]-4- Formamide (Compound A-2)
[0139]
[0140] With reference to the method of Example 1, 3-bromobenzenesulfonyl chloride was replaced by 2-methoxy-5-bromobenzenesulfonyl chloride to obtain compound A-2: 1 H NMR (300MHz, DMSO-d 6)δppmδ10.10(s,1H),9.38(s,1H),9.27(s,1H),8.05(d,J=2.3Hz,2H),8.02(s,1H),7.97(dd,J=8.6 ,2.3Hz,1H),7.87-7.73(m,4H),7.70(d,J=2.2Hz,1H),7.42(dd,J=8.8,2.4Hz,1H),7.32-7.38(m,3H) ,7.17(s,2H),6.70(d,J=8.8Hz,1H),3.97(s,3H).ESI-MS:m / z 570.1[M-H] - .
Embodiment 3
[0142] 4'-(3-((4-fluorophenyl)sulfonylamino)-4-hydroxyphenyl)carbamoyl)-[1,1'-biphenyl]-4-carboxylic acid methyl ester (Compound A -3)
[0143]
[0144] Referring to the method of Example 1, replace I-3 with 4'-(methoxycarbonyl)-[1,1'-biphenyl]-4-carboxylic acid to obtain compound A-3: 1 H NMR (300MHz, DMSO-d 6 )δppm 10.15(s,1H),9.41(s,1H),9.31(s,1H),8.11-8.03(m,4H),7.91(m,4H),7.82(dd,J=8.8,5.2Hz, 2H), 7.71(d, J=2.2Hz, 1H), 7.43(dd, J=8.6, 2.2Hz, 1H), 7.33-7.39(m, 2H), 6.70(d, J=8.7Hz, 1H), 3.89(s,3H).ESI-MS: m / z519.1[M-H] - .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com