Novel adeno-associated virus (AAV) vectors, aav vectors having reduced capsid deamidation and uses therefor
A deamidation and capsid technology, applied in isoaspartic acid, asparagine is deamidated to aspartic acid, mutual transfer, molecular field, can solve problems such as complex drugs and poorly understood strategies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
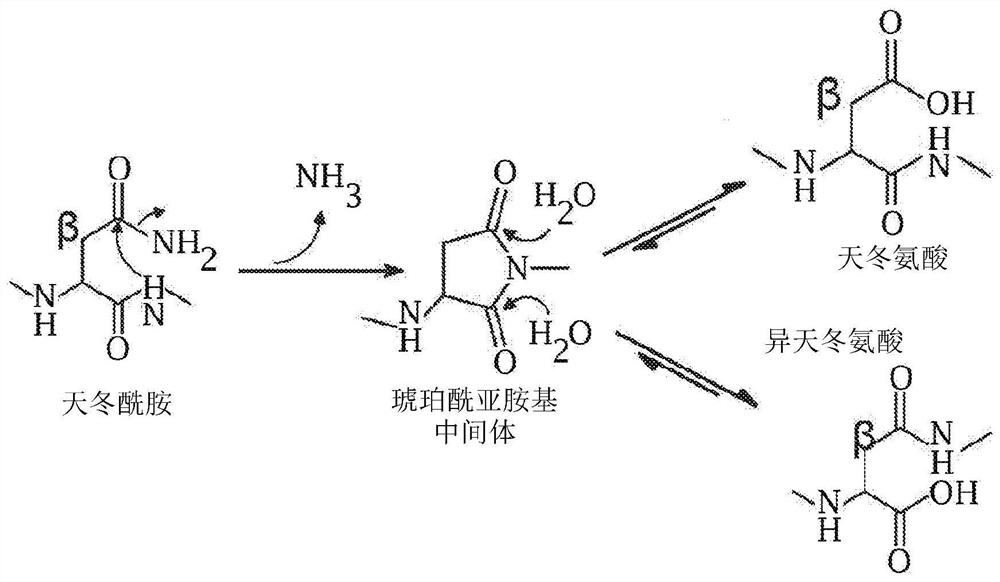
[0202]The following examples report extensive deamidation of AAV8 and 7 additional different AAV serotypes and supporting evidence from structural, biochemical, and mass spectrometry methods. The degree of deamidation at each site depends on the age of the vector and a number of main sequence and 3D structural factors, but it is largely not restricted by the conditions of vector recovery and purification. It is proved that deamidation may affect vector transduction activity, and the loss of the early time point of vector activity is related to the spontaneous deamidation that progresses rapidly at several AAV8 asparagines. A mutation strategy for stabilizing side chain amides was explored to improve vector transduction and reduce batch-to-batch molecular variability, which is a key issue in biological preparation. This study demonstrates previously unknown aspects of AAV capsid heterogeneity and highlights its importance in the development of these vectors for gene therapy.
[0203]The...
example 4
[0204]Example 4 relates to a novel epitope mapped on the AAV9 capsid.
example 1
[0205]Example 1: Deamidation of amino acids on the surface of the adeno-associated virus capsid
[0206]A. Materials and methods
[0207]1.1D and 2D gel electrophoresis
[0208]In order to perform 1D SDS polyacrylamide gel electrophoresis (SDS-PAGE) analysis, the AAV vector was first denatured at 80°C for 20 minutes in the presence of lithium dodecyl sulfate and a reducing agent. Then, it was run on a 4-12% Bis-Tris gel at 200V for 90 minutes and stained with coomassie blue. forFigure 1A-Figure 1DThe data in Kendrick Laboratories, Inc. (Madison, Wisconsin) performed 2D gel electrophoresis. For follow-up experiments, 2D SDS-PAGE was performed internally. To this end, 3×1011GC's AAV vector and 500Uturbonuclease marker (Accelagen, San Diego, CA) in 150μL containing 35mM NaCl and 1mM MgCl2The phosphate buffered saline (PBS) was combined together and incubated at 37°C for ten minutes. Next, add nine volumes of absolute ethanol, vortex the sample, and then incubate it at -80°C for at least two hou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com