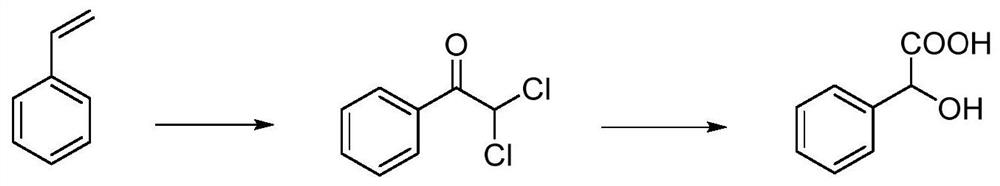
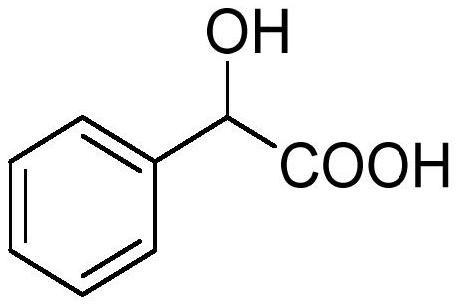
Method for synthesizing mandelic acid
A technology of mandelic acid and mixed solution is applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylates, etc., can solve the problems of backward adopting methods, large environmental pollution, high price, etc., and achieves short production process flow, The effect of low waste discharge and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]S1. Add styrene, ethylene glycol dimethyl ether and water into a three-necked flask, add trichloroisocyanuric acid, heat up the reaction to the end, cool the reaction solution to room temperature, filter, and recover the filtered white solid for use. The filtrate was extracted with dichloromethane, the organic layer was collected, and the solvent was evaporated under reduced pressure to obtain 2,2-dichloroacetophenone.
[0034]S2. Under stirring conditions, hydrolyze 2,2-dichloroacetophenone in a sodium hydroxide solution, adjust the pH to 2-3 with hydrochloric acid, and cool to precipitate mandelic acid.
Embodiment 2
[0036]S1. Dissolve 1.04g (10mmol) of styrene in a mixed solution of ethylene glycol dimethyl ether and water, slowly add 1.3eq of trichloroisocyanuric acid under ice bath conditions, heat to 70°C, continue to stir and react To the end of the reaction. The reaction solution was cooled to room temperature, filtered, the filtrate was added, extracted with dichloromethane, filtered, and spin-dried to obtain 1.31 g of 2,2-dichloroacetophenone. The white solid cyanuric acid was filtered out and recovered, with a recovery rate of 95%.
[0037]S2. Take 10ml of sodium hydroxide solution with a mass fraction of 10%, and slowly add the crude product in step (2) dropwise. The temperature is controlled at 50°C and the temperature is kept for one hour. Then the pH is adjusted to 1-2 with hydrochloric acid, and then added 0.1g of activated carbon faded, and after keeping it for one hour, 1.06g of crystals precipitated.
Embodiment 3
[0039]S1. Dissolve 1.04g (10mmol) of styrene in a mixed solution of ethylene glycol dimethyl ether and water, slowly add 1.5eq of trichloroisocyanuric acid under ice bath conditions, heat to 70°C, continue to stir and react To the end of the reaction. The reaction solution was cooled to room temperature, filtered, the filtrate was added, extracted with dichloromethane, filtered, and spin-dried to obtain 1.59 g of 2,2-dichloroacetophenone. The white solid cyanuric acid was filtered out and recovered, with a recovery rate of 95%.
[0040]S2. Take 10ml of sodium hydroxide solution with a mass fraction of 10%, and slowly add the crude product in step (1) dropwise. The temperature is controlled at 50°C and kept for one hour. Then the pH is adjusted to 1-2 with hydrochloric acid and added 0.1g of activated carbon faded, and after holding for one hour, 1.27g of crystals precipitated.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com