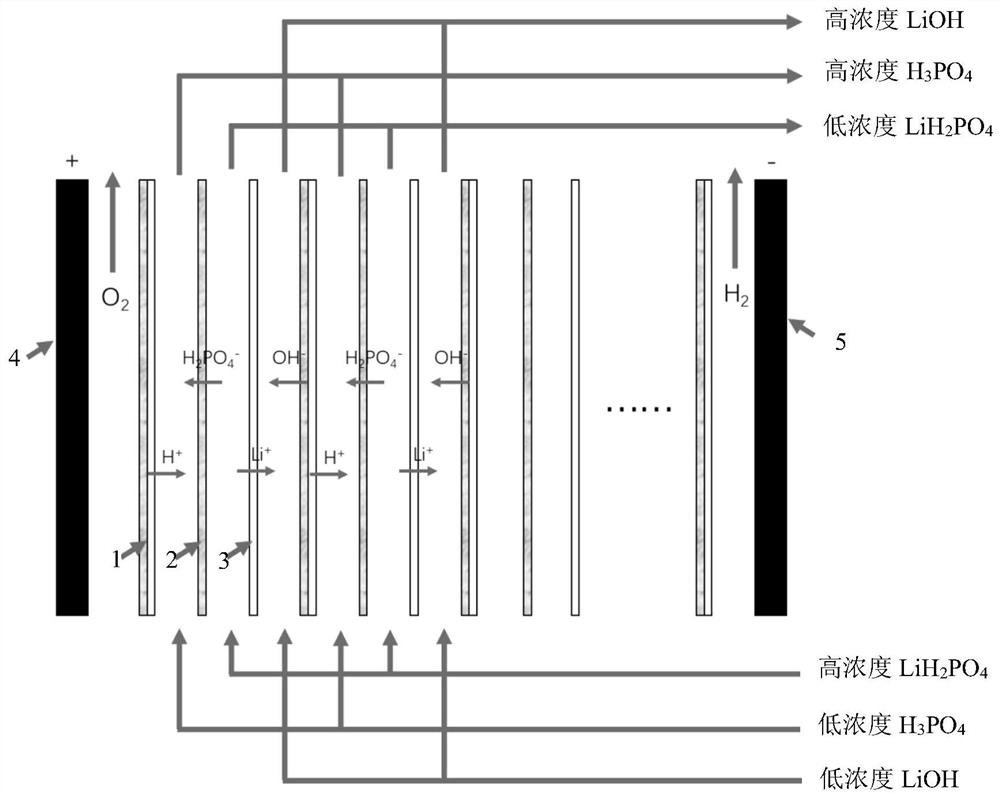
Method for producing lithium hydroxide from lithium-containing low-magnesium brine in form of lithium phosphate
A lithium hydroxide and lithium phosphate technology, applied in the direction of lithium oxide;/hydroxide, etc., can solve the problems of reducing electrolysis production capacity, increasing electrolysis cost, and high concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The composition of the brine in Zabuye Salt Lake in Tibet is shown in Table 1. The pH value of the salt lake brine is 9.2 and the density is 1.24g / cm 3 .
[0053] Table 1 The brine composition of Zabuye Salt Lake
[0054] Element K +
Ca 2+
Mg 2+
Cl -
CO 3 2-
SO 4 2-
B 2 o 3
Li +
Na +
Concentration(g / L) 31.6 0.002 0.02 120.6 34.1 29.8 8.4 0.81 100.1
[0055] Take 100L of the salt lake brine sample, add lithium hydroxide mother liquor to adjust the pH to 11, filter and remove the resulting precipitate, and obtain the brine sample after calcium and magnesium removal, and use plasma inductively coupled atomic emission spectrometry (ICP) to analyze the lithium in it The concentration was 0.79g / L, and the calcium and magnesium content was analyzed at the same time. The Mg and Ca in the solution were both lower than the ICP-AES inspection limit, indicating that calcium and magnesium had been c...
Embodiment 2
[0061] The main composition of an underground brine in Jiangxi is (g / L): Li + , 0.097; Mg 2+ , 0.929; Na + , 120.6; K + , 0.421; Ca 2+ , 4.045; Cl - , 196.7, use the adsorption method of aluminum-based adsorbent to remove magnesium and concentrate lithium to prepare a desorption solution containing lithium and low magnesium. The main composition of the desorption solution is (g / L): Li + , 0.23; Mg 2+ , 0.004; Na + , 0.43; K + , 0.002; Ca 2+ , 0.015; Cl - , 1.88, further reverse osmosis of the desorption solution to obtain a concentrated solution containing lithium and low magnesium, the concentrated solution is mainly composed of (g / L): Li + , 2.3; Mg 2+ , 0.04; Na + , 4.3; K + , 0.02; Ca 2+ , 0.15; Cl - , 18.8.
[0062] Take 100L of the above-mentioned concentrated solution, add lithium hydroxide lithium precipitation mother liquor and sodium hydroxide solution to adjust the pH value to 11.5, filter and remove the precipitate produced, and obtain a brine sample...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com