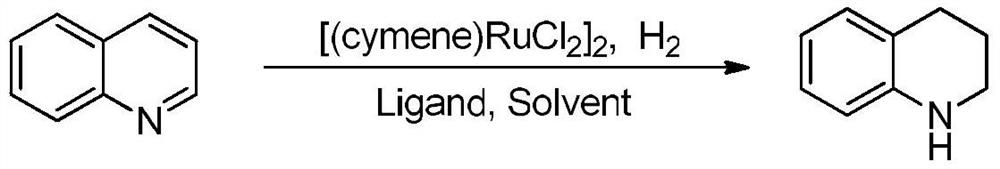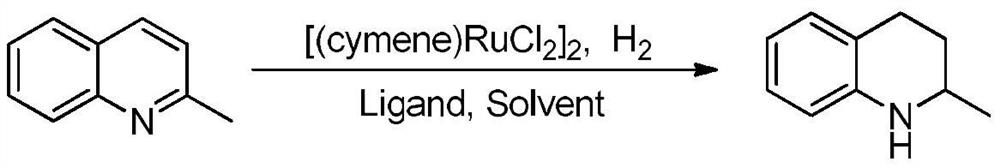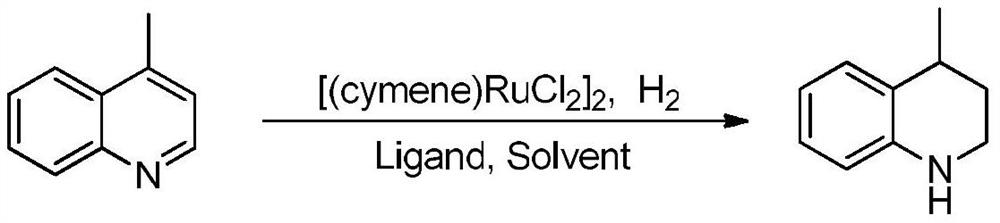Method for preparing tetrahydroquinoline compounds by catalytic hydrogenation of ruthenium catalyst
A technology of catalytic hydrogenation and tetrahydroquinoline, which is applied in the field of synthetic chemistry, can solve the problems of discharging a large amount of silicon-containing waste, low atom economy, and high price of silane, and achieves a simple and green synthesis method, high reaction atom economy, and highly selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A method for preparing tetrahydroquinoline compounds by catalytic hydrogenation of a ruthenium catalyst, the reaction formula is as follows:
[0028]
[0029] The catalyst [(cymene)RuCl 2 ] 2 (0.03mmol), phosphine ligand triphenylphosphine PPh 3 (0.07mmol), quinoline (1.0mmol), then add solvent methanol 2mL, pump and exchange air three times under hydrogen atmosphere, connect hydrogen balloon under normal pressure, react at room temperature for 8 hours, and concentrate by vacuum rotary evaporation after the reaction The reaction solution was separated by column chromatography to obtain the corresponding product with a separation yield of 90%, high selectivity and no other by-products. 1 H NMR (400MHz, CDCl 3 )δ:6.97-6.93(m,2H),6.60(td,J=7.2,1.2Hz,1H),6.46(d,J=7.8Hz,1H),3.73(s,1H),3.30-3.28(m ,2H),2.76(t,J=6.0Hz,2H),1.96-1.92(m,2H);HRMS(ESI):calcd for C 9 h 12 N[M+H] + 134.2012, found 134.2013.
Embodiment 2
[0031] A method for preparing tetrahydroquinoline compounds by catalytic hydrogenation of a ruthenium catalyst, the reaction formula is as follows:
[0032]
[0033] The catalyst [(cymene)RuCl 2 ] 2 (0.05mmol), phosphine ligand tricyclohexylphosphine PCy 3 (0.12mmol), 2-methylquinoline (1.0mmol), and then add solvent ethanol 2mL, pump and change air three times under hydrogen atmosphere, connect hydrogen balloon under normal pressure, react at room temperature for 10 hours, and use reducing The reaction solution was concentrated by rotary evaporation, and the corresponding product was obtained by column chromatography separation, with an isolated yield of 93%, high selectivity, and no other by-products. 1 H NMR (400MHz, CDCl 3 )δ:6.96-6.94(m,2H),6.59(td,J=7.2,1.2Hz,1H),6.45(dd,J=7.2,1.2Hz,1H),3.67(s,1H),3.41-3.37 (m,1H),2.85-2.80(m,1H),2.74-2.70(m,1H),1.93-1.89(m,1H),1.59-1.56(m,1H),1.19(d,J=6.0Hz ,3H); HRMS(ESI):calcd for C 10 h 14 N[M+H] + 148.1126, found 148.1123...
Embodiment 3
[0035] A method for preparing tetrahydroquinoline compounds by catalytic hydrogenation of a ruthenium catalyst, the reaction formula is as follows:
[0036]
[0037] The catalyst [(cymene)RuCl 2 ] 2 (0.04mmol), phosphine ligand trimethylphosphine PMe 3(0.10mmol), 4-methylquinoline (1.0mmol), and then add solvent methanol 2mL, pump and change air three times under hydrogen atmosphere, connect a hydrogen balloon under normal pressure, and react at room temperature for 12 hours. The reaction solution was concentrated by rotary distillation, and the corresponding product was obtained by column chromatography separation with an isolated yield of 94%, high selectivity and no other by-products. 1 H NMR (400MHz, CDCl 3 )δ:7.05(d,J=7.8Hz,1H),6.97-6.94(m,1H),6.63(td,J=7.2,0.6Hz,1H),6.46(d,J=7.8Hz,1H), 3.85(s,1H),3.35-3.31(m,1H),3.29-3.25(m,1H),2.94-2.88(m,1H),2.01-1.96(m,1H),1.70-1.65(m,1H ), 1.29 (d, J=7.2Hz, 3H); HRMS (ESI): calcd for C 10 h 14 N[M+H] + 148.1126, found 148....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com