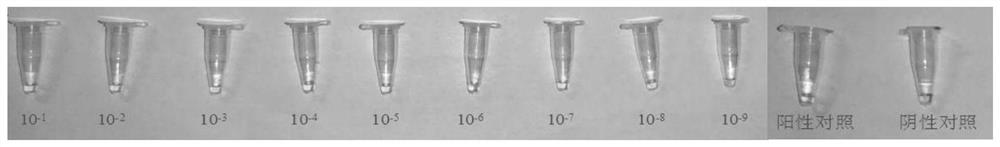
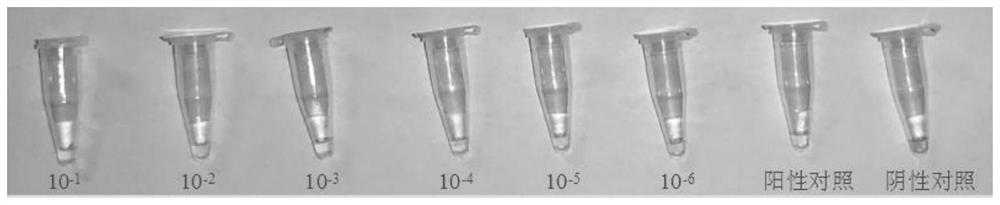
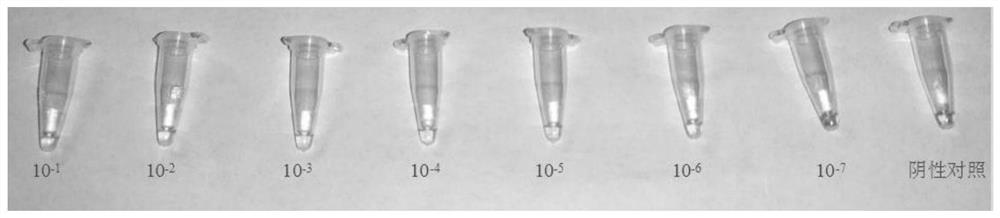
Virus sample treating fluid and treating method of novel coronavirus and rapid constant-temperature reverse transcription amplification kit for detecting virus
A sample processing liquid and coronavirus technology, applied in the direction of microorganism-based methods, biochemical equipment and methods, microorganism measurement/inspection, etc., can solve the problems of time-consuming, loss, and cost of nucleic acid extraction steps, and achieve nucleic acid detection Easy, small reaction volume, cost-saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Virus inactivation method
[0082] Viruses are inactivated in two ways:
[0083] Method 1: Inactivate the virus by chemical method with a final concentration of 0.2M guanidine hydrochloride. The specific implementation process is as follows: take 960 μL of clinically collected virus sample solution, add 40 μL of 5.0 M guanidine hydrochloride solution, shake and mix for 10 seconds, and centrifuge at 13000 rpm for 1.5 minutes to obtain the processed sample to be tested.
[0084] Method 2 uses 0.1xTE buffer solution with a final mass percentage of Tween-20 of 0.01% to chemically inactivate the virus. The specific implementation process is as follows: take 960 μL of the clinically collected virus sample solution, add 40 μL of 0.1xTE buffer solution containing 0.25% Tween-20 by mass, treat it in a water bath at 95°C for 10 minutes, cool it, and centrifuge it at 13000rpm for 1.5min. The processed sample to be tested. The tube cap can only be opened after the inactivated vi...
Embodiment 2
[0086] Virus inactivation method
[0087] Viruses are inactivated in two ways:
[0088] Method 1: Inactivate the virus by chemical method with a final concentration of 0.4M guanidine hydrochloride. The specific implementation process is as follows: take 920 μL of clinically collected virus sample solution, add 80 μL of 5.0 M guanidine hydrochloride solution, shake and mix for 10 seconds, and centrifuge at 13,000 rpm for 1.5 minutes to obtain the processed sample to be tested.
[0089] Method 2 uses 0.1xTE buffer with a final mass percentage of Tween-20 of 0.1% to chemically inactivate the virus. The specific implementation process is as follows: take 600 μL of clinically collected virus sample solution, add 400 μL of 0.1xTE buffer solution containing 0.25% Tween-20 by mass, treat it in a water bath at 95°C for 10 minutes, cool it, and centrifuge it at 13000rpm for 1.5min. The processed sample to be tested. The tube cap can only be opened after the inactivated virus is coole...
Embodiment 3
[0091] Virus inactivation method
[0092] Viruses are inactivated in two ways:
[0093] Method 1: Use the chemical method to inactivate the virus with a final concentration of 0.6M guanidine hydrochloride. The specific implementation process is as follows: take 880 μL of clinically collected virus sample solution, add 120 μL of 5.0 M guanidine hydrochloride solution, shake and mix for 10 seconds, and centrifuge at 13000 rpm for 1.5 minutes to obtain the processed sample to be tested.
[0094] Method 2 uses 0.1xTE buffer solution with a final mass percentage of Tween-20 of 0.05% to chemically inactivate the virus. The specific implementation process is as follows: take 800 μL of clinically collected virus sample solution, add 200 μL of 0.1xTE buffer solution containing 0.25% Tween-20 by mass, treat it in a water bath at 95°C for 10 minutes, cool it, and centrifuge it at 13000rpm for 1.5min. The processed sample to be tested. The tube cap can only be opened after the inactiva...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com