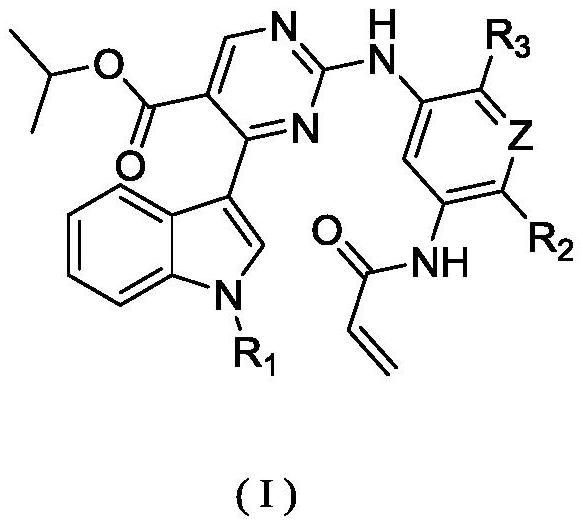
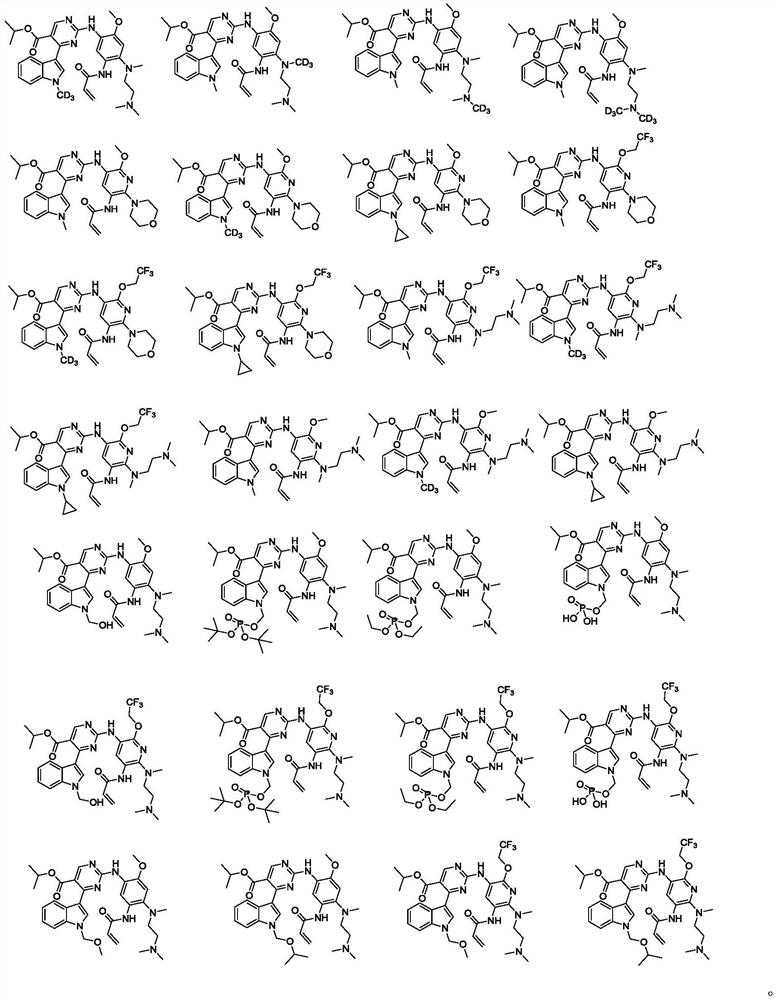
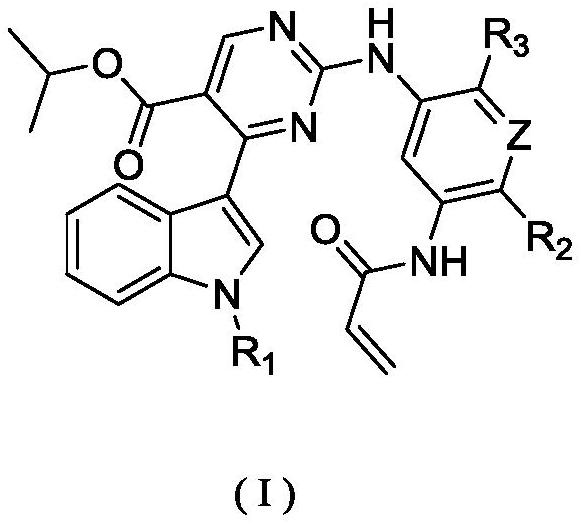
Substituted pyrimidine compound, pharmaceutical composition thereof and use of the compound
A compound and application technology, applied in the field of chemical medicine, to achieve the effect of reducing rash and diarrhea, improving diseases and disorders, and high metabolic stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0103]
[0104] Another aspect of the present invention also provides a method for preparing a compound of general formula (I), which is implemented by one of the following reaction schemes:
[0105] Reaction scheme one:
[0106]
[0107] Reaction 1
[0108] As shown in Reaction Formula 1, using isopropyl 2,4-dichloropyrimidine-5-carboxylate as a raw material, reacting with compound A under the action of a catalyst to obtain intermediate a through Friedel-Crafts reaction; intermediate a is substituted with compound B Reaction obtains compound C; Wherein, R 1 selected from hydrogen, C 1 -C 4 Alkyl, deuterated methyl, C 3 -C 6 Cycloalkyl, R 2 , R 3 , the definition of Z is identical with the definition in general formula (I),
[0109] In the above reaction, the preparation of the intermediate a is carried out under the action of a Lewis acid, and the Lewis acid can be selected from, but not limited to, iron trichloride, aluminum trichloride, zinc chloride, trifluori...
Embodiment 1
[0219] Example 1: Isopropyl-2-((5-acrylamido-4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxyphenyl)amino) -4-(1-Deuteromethyl-1H-indol-3-yl)pyrimidine-5-carboxylate
[0220]
[0221] Intermediate 2b (24mg, 0.073mmol) was dissolved in acetonitrile (2mL), and intermediate 1a (25mg, 0.087mmol) and p-toluenesulfonic acid monohydrate (4mg, 0.022mmol) were added successively, under nitrogen protection, heated to 80 °C for 18 hours. After the reaction solution was concentrated, it was purified by column to obtain 15 mg of gray solid with a yield of 35.3%.
[0222] ESI-MS m / z:589.4[M+H] + , 1 H-NMR (DMSO-d 6 ,400MHz),10.12(s,1H),8.84(s,1H),8.64(d,J=8.0Hz,2H),8.17(s,1H),7.73(s,1H),7.48(d,J= 8.0Hz,1H),7.19(t,J=8.0Hz,1H),7.05-7.01(m,2H),6.46-6.40(m,1H),6.29-6.20(m,1H),5.77(d,J =8.0Hz,1H),5.03-4.96(m,1H),3.87(s,3H),2.89(t,J=8.0Hz,2H),2.72(s,3H),2.32(t,J=8.0Hz ,2H),2.21(s,6H),1.12(d,J=8.0Hz,6H).
Embodiment 1A
[0223] Example 1A: Isopropyl-2-((5-acrylamido-4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxyphenyl)amino) -4-(1-Deuteromethyl-1H-indol-3-yl)pyrimidine-5-carboxylate succinate
[0224]
[0225] Isopropyl-2-((5-acrylamido-4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxyphenyl)amino)-4- (1-Deuteromethyl-1H-indol-3-yl)pyrimidine-5-carboxylate (400mg, 0.68mmol) in tetrahydrofuran (4mL) was slowly added dropwise with succinic acid (84.2mg, 0.71mmol) Tetrahydrofuran (1 mL) solution. Keep stirring for 12 hours. The solid precipitated, was filtered while it was hot, and dried in vacuo to obtain 320 mg of a light yellow solid with a yield of 66.7%.
[0226] ESI-MS m / z:589.4[M+H] + , 1 H-NMR (DMSO-d 6 ,400MHz),10.11(s,1H),8.83(s,1H),8.62(d,J=8.0Hz,2H),8.17(s,1H),7.73(s,1H),7.46(d,J= 8.0Hz,1H),7.18(t,J=8.0Hz,1H),7.05-6.09(m,2H),6.46-6.41(m,1H),6.29-6.20(m,1H),5.77(d,J =8.0Hz,1H),5.03-4.96(m,1H),3.87(s,3H),2.89(t,J=8.0Hz,2H),2.72(s,3H),2.41(s,2H),2.32 (t,J=8.0Hz,2H),2.21(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com