Tetrahydropyrrolooxazinone and piperidino-oxazinone compounds and preparation method thereof
A technology of tetrahydropyrrole and oxazinone, which is applied in the field of tetrahydropyrroloxazinone and piperidoxazinone compounds and their preparation, can solve problems such as adverse effects on human health and the environment, urgent problems, etc., and achieve a simple route , simple operation, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
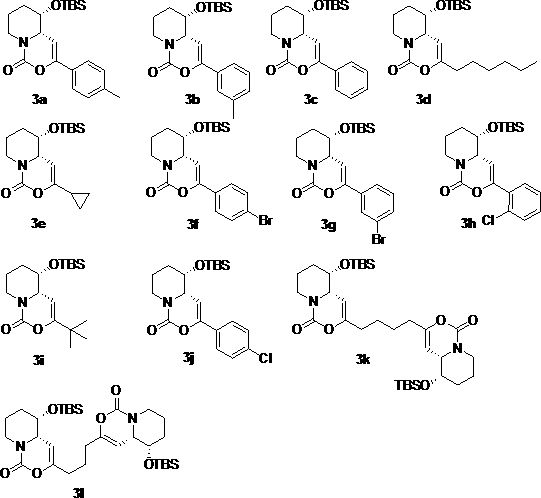
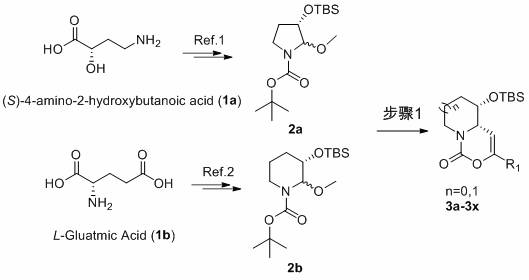
[0032] Synthesis of compound 3a
[0033] (4a S ,5 S )-5-( tert -Butyldimethylsilyloxy)-3- p -tolyl-5,6,7,8-tetrahydropyrido[1,2-c][1,3]oxazin-1(4a H )-one (3a)
[0034] -50 o Under the protection of C nitrogen, slowly drop boron trifluoride ether into compound 2b and dichloromethane solution of p-methylphenylacetylene to react for 0.5-5 hours, then naturally rise to room temperature, add aqueous solution of sodium bicarbonate, and react at room temperature for 5-10 minutes , extracted, dried over anhydrous sodium sulfate, filtered with suction, concentrated, and purified on a silica gel column to obtain white solid 3a (78 mg, 72%). 1 H NMR (400 MHz, CDCl 3 ) δ 7.53-7.46 (m, 2H), 7.20-7.12 (m, 2H), 5.31-5.22 (m, 1H), 4.46 (d, J =13.2 Hz, 1H), 4.01-3.95 (m, 1H), 3.76-3.69 (m, 1H), 2.82-2.71 (m, 1H), 2.34(s, 3H), 2.16-2.04 (m, 1H), 1.94-1.86 (m, 1H), 1.69-1.60 (m, 1H), 1.47-1.40(m, 1H), 0.79 (s, 9H), 0.01 (s, 3H), -0.11 (s, 3H) ppm.
[0035] Synthesis of compound 3b
...
Embodiment 2
[0102] Synthesis of compound 3a
[0103] (4a S ,5 S )-5-( tert -Butyldimethylsilyloxy)-3- p -tolyl-5,6,7,8-tetrahydropyrido[1,2-c][1,3]oxazin-1(4a H )-one (3a)
[0104] -50 o Under nitrogen protection, slowly add copper trifluoromethanesulfonate to compound 2b and react with dichloromethane solution of p-methylphenylacetylene for 0.5-5 hours, then naturally rise to room temperature, add aqueous solution of sodium bicarbonate, and react at room temperature for 5-10 minutes , extracted, dried over anhydrous sodium sulfate, filtered with suction, concentrated, and purified on a silica gel column to obtain a white solid 3a (52 mg, 48%).
Embodiment 3
[0106] Synthesis of compound 3a
[0107] (4a S ,5 S )-5-( tert -Butyldimethylsilyloxy)-3- p -tolyl-5,6,7,8-tetrahydropyrido[1,2-c][1,3]oxazin-1(4a H )-one (3a)
[0108] -50 o Under the protection of C nitrogen, slowly add trifluoroacetic acid to compound 2b and react with the dichloromethane solution of p-methylphenylacetylene for 0.5-5 hours, then naturally rise to room temperature, add aqueous solution of sodium bicarbonate, react at room temperature for 5-10 minutes, and extract , dried over anhydrous sodium sulfate, filtered with suction, concentrated, and purified on a silica gel column to obtain a white solid 3a (11 mg, 10%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com