Continuous acylation synthesis method in acesulfame potassium synthesis
A technology for the synthesis of acesulfame potassium, which is applied in the chemical industry, can solve problems such as difficult stability of product quality, and achieve the effects of solving unstable quality and potential safety hazards, low overall cost, and reducing labor intensity and equipment investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
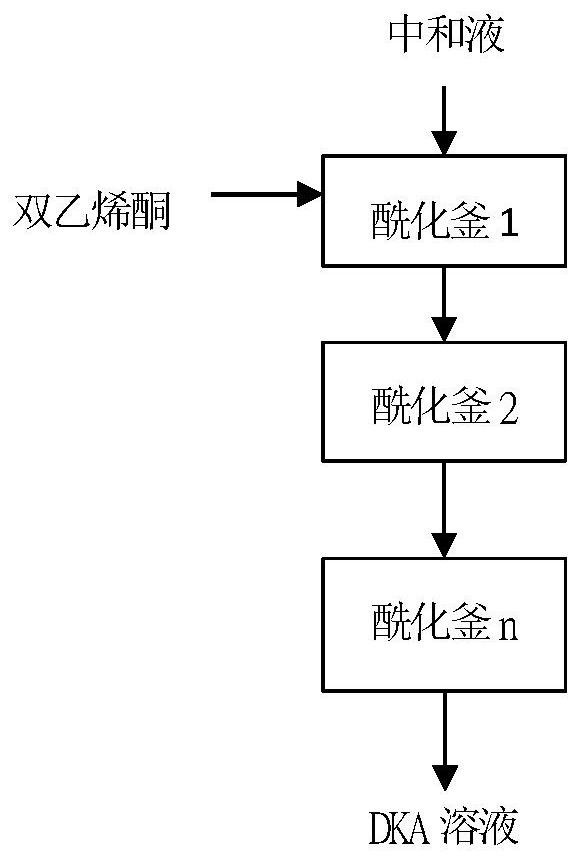
[0041] Add 600g of neutralization reaction solution (containing 0.76mol of sulfamic acid) into the acylation kettle 1, then start stirring, and slowly add 78.2g of diketene ketone dropwise. During the process, the reaction temperature is controlled at 20-25°C, and the dropwise addition time is 40min. After the addition of diketene ketone is completed Finally, keep warm at 20-25°C for 1.5h to complete the preparation of the bottoming solution.
[0042] The neutralization reaction solution 30g / h and diketene 3.9g / h are continuously fed into the acylation kettle 1; the residence time of each acylation kettle is 1h, and it overflows to the acylation kettle 2 by means of overflow, and the acylation kettle 2 passes through The overflow method overflows to the acylation kettle 3, and the acylation kettle 3 overflows to the acylation kettle 4 by means of overflow, and the residence time is 4 hours in total. During the process, the temperature of each acylation kettle is controlled at 2...
Embodiment 2
[0044] Add 600g of neutralization reaction solution (containing 0.76mol of sulfamic acid) into the acylation kettle 1, then start stirring, and slowly add 71.66g of diketene ketone dropwise. During the process, the reaction temperature is controlled at 25-30°C, and the dropwise addition time is 20min. The addition of diketene ketone is completed. Finally, keep warm at 20-25°C for 1 hour to complete the preparation of the bottoming solution.
[0045]The neutralization reaction solution 30g / h and diketene 3.5g / h are continuously fed into the acylation kettle 1; the residence time of each acylation kettle is 1h, and it overflows to the acylation kettle 2 by means of overflow, and the acylation kettle 2 passes through The way of overflow overflows to the acylation kettle 3, and the residence time is 3 hours in total. During the process, the temperature of each acylation kettle is controlled at 25-35°C, and the acylation kettle 3 overflows to the receiving kettle by overflowing, and...
Embodiment 3
[0047] Add 600g of neutralization reaction solution (containing 0.76mol of sulfamic acid) into the acylation kettle 1, then start stirring, and slowly add 65.2g of diketene ketone dropwise. During the process, the reaction temperature is controlled at 25-30°C, and the dropwise addition time is 20min. The addition of diketene ketone is complete Finally, keep warm at 20-25°C for 1 hour to complete the preparation of the bottoming solution.
[0048] 30g / h of the neutralization reaction solution and 2.5g / h of diketene are continuously fed into the acylation kettle 1; the residence time of each acylation kettle is 1h, and it overflows to the acylation kettle 2 by means of overflow, and the acylation kettle 2 passes through The way of overflow overflows to the acylation kettle 3, and the residence time is 3 hours in total. During the process, the temperature of each acylation kettle is controlled at 25-35°C, and the acylation kettle 3 overflows to the receiving kettle by overflowing,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com