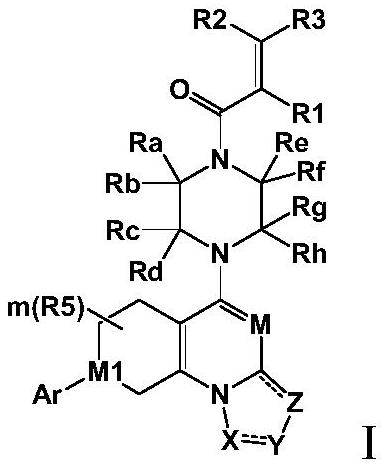
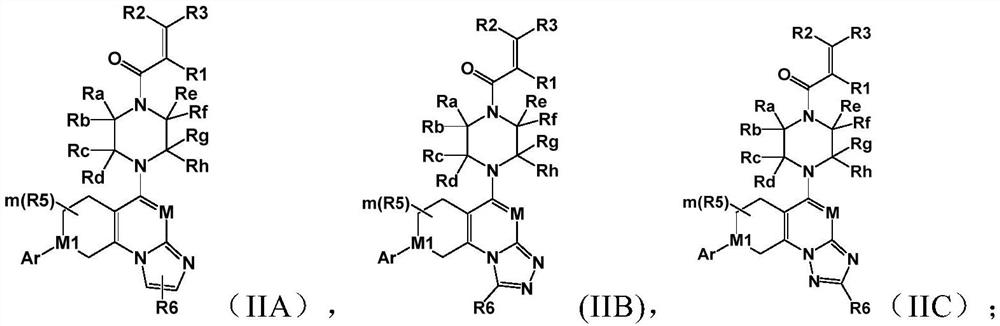
Saturated six-membered ring heterocyclic compound as well as preparation method and application thereof
A technology of compounds and solvates, applied in the field of medicinal chemistry, can solve the problems of unsuccessful development of targeted drugs and low efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0202] Example 1: (S)-1-(4-(8-(5-methyl-1H-indazol-4-yl)-1-(1-methylpyrrolin-2-yl)-6,7 ,8,9-Tetrahydropyrido[4,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5-yl)piperazin-1-yl)propan-2 - en-1-one
[0203]
[0204] The first step: tert-butyl 2,4-dichloro-5,8-dihydropyrido[3,4-d]pyrimidine-7(6H)-carboxylate (10.4g, 34.3 mmol) and piperazine- Benzyl 1-carboxylate (7.5 g, 34.1 mmol) was dissolved in 1,4-dioxane (200 mL), and DIPEA (13.2 g, 102 mmol) was added. React at room temperature for 2 days, concentrate under reduced pressure, and purify the residue by column chromatography to obtain 4-(4-((benzyloxy)carbonyl)piperazin-1-yl)-2-chloro-5,8-dihydropyrido[ 3,4-d]pyrimidine-7(6H)-carboxylate tert-butyl ester (11 g, white solid). LC-MS:ESI[M+H] + =488.5; 1 HNMR (400MHz, DMSO-d 6 ): δ7.33-7.39 (m, 5H), 5.11 (s, 2H), 4.37 (brs, 2H), 3.50 (brs, 10H), 2.63-2.65 (m, 2H), 1.44 (s, 9H).
[0205] The second step: 4-(4-((benzyloxy)carbonyl)piperazin-1-yl)-2-chloro-5,8-dihydropyrido[3,4-d]...
Embodiment 2
[0214] Example 2: 1-(4-(8-(5-methyl-1H-indazol-4-yl)-6,7,8,9-tetrahydropyrido[4,3-e][1, 2,4] Triazolo[4,3-a]pyrimidin-5-yl)piperazin-1-yl)prop-2-en-1-one
[0215]
[0216] The first step: 4-(2-hydrazino-7-(5-methyl-1-((2-(trimethylsilyl)ethoxy)methyl)-1H-indazol-4-yl )-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-4-yl)piperazine-1-carboxylate benzyl ester (0.5g, 0.79mmol) was dissolved in dichloromethane (15mL ), trimethyl orthoformate (340mg, 3.2mmol) was added, and after stirring for ten minutes, trifluoroacetic acid (95mg, 0.83mmol) was added. After continuing to stir at room temperature for 1 hour, it was concentrated under reduced pressure, and the residue was separated and purified by silica gel column chromatography to obtain 4-(8-(5-methyl-1-((2-(trimethylsilyl)ethoxy)methoxy)methanol Base)-1H-indazol-4-yl)-6,7,8,9-tetrahydropyrido[4,3-e][1,2,4]triazol[4,3-a]pyrimidine -5-yl)benzylpiperazine-1-carboxylate (320 mg, yellow solid). LC-MS:ESI[M+H] + = 654.7; 1 H NMR(4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com