Preparation method of freeze-dried human blood coagulation factor VIII
A human coagulation factor and freeze-drying technology, which is applied in the preparation method of peptides, coagulation/fibrinolytic factors, factor VII, etc., can solve the problems of lack of plasma resources, scientific research technology, long reconstitution time, limited quantity, etc., and achieve the goal of improving plasma The effect of resource utilization, improving purity and specific activity, and alleviating medication tension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
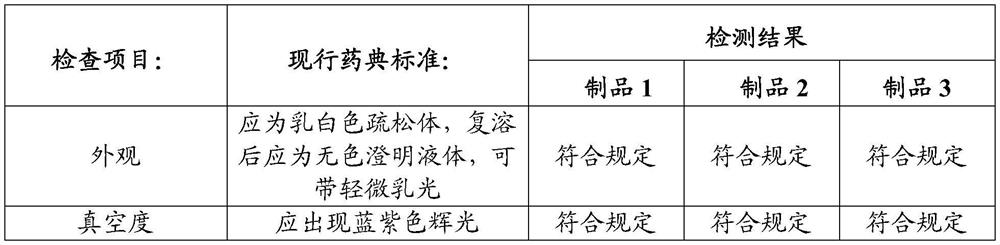
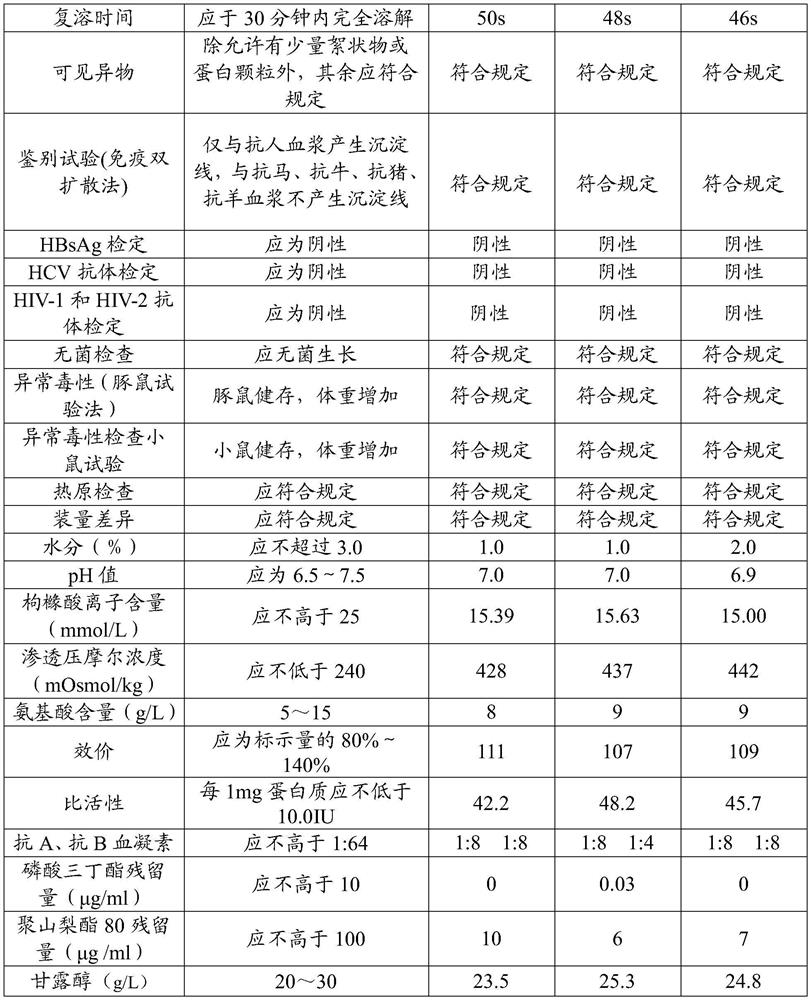
Examples
preparation example Construction
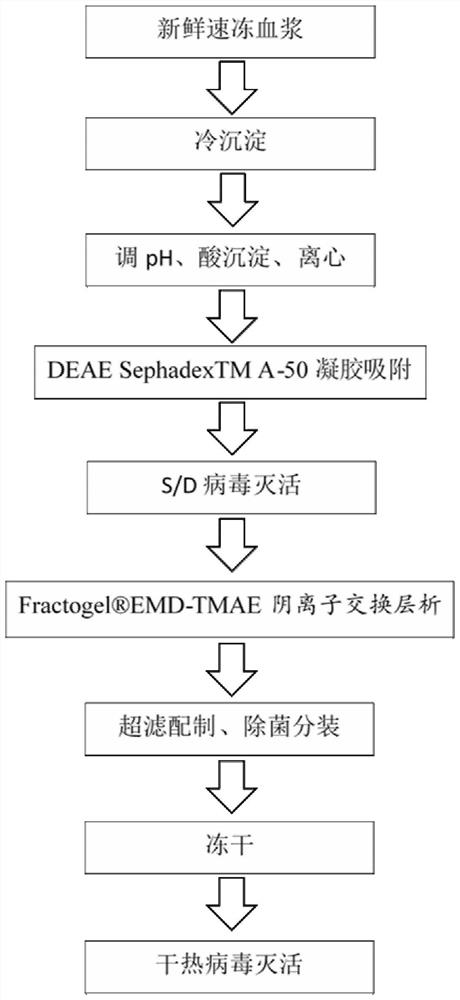
[0097] Such as figure 1 Shown, a kind of lyophilized human blood coagulation factor VIII preparation method of the present invention comprises the following steps:
[0098] (1) Collection of plasma and quick-frozen plasma;
[0099] (2) Plasma thawing and separation of cryoprecipitate: pre-thawing the quick-frozen plasma; then thawing the plasma and centrifuging to obtain cryoprecipitate, which is the raw material of human blood coagulation factor VIII;
[0100] (3) cryoprecipitate dissolving: the cryoprecipitate obtained in step (2) is dissolved, and the pH is adjusted;
[0101] (4) Acid precipitation: add acetic acid buffer to the cryoprecipitate lysate in step (3) to adjust the pH and centrifuge to obtain the supernatant;
[0102] (5) Gel adsorption: the supernatant after step (4) was centrifuged, and the pH was adjusted with sodium hydroxide buffer. Then add DEAE SephadexTM A-50 gel for adsorption, after adsorption, filter with a filter element to obtain filtrate A;
[...
Embodiment 1
[0112](1) Plasma collection and quick-freezing: 3.49 tons of fresh human plasma collected was quick-frozen within 30 minutes by flat-plate direct-cooling quick-freezing technology and stored in a -30°C freezer. This batch of plasma passed the virus test and met the quarantine period stipulated by the state.
[0113] (2) Plasma melting and cryoprecipitation: transfer the batch of qualified plasma from the -30°C freezer to the pre-thawing room at 0-4°C for 1 hour, and then spray it with water for injection below 10°C. Alcohol spray disinfection and water for injection rinse alcohol and dry. Break the bag of the raw material plasma and transfer it to the slurry tank, and circulate the water for injection with an interlayer temperature of 30°C to melt the plasma, and perform continuous separation and centrifugation at a speed of 5000-5600 rpm. kg and supernatant.
[0114] (3) Cryoprecipitate dissolving: the 18.0kg cryoprecipitate obtained in step (2) is cut into pieces, and diss...
Embodiment 2
[0137] (1) Plasma collection and quick-freezing: 3.50 tons of fresh human plasma was collected and frozen within 30 minutes by direct-cooling and quick-freezing technology on a plate and stored in a -30°C freezer. This batch of plasma passed the virus test and met the quarantine period stipulated by the state.
[0138] (2) Plasma melting and cryoprecipitation: transfer the batch of qualified plasma from the -30°C cold storage to the pre-thawing room at 0-4°C for 1.5 hours, and then spray it with water for injection below 10°C. Alcohol spray disinfection and water for injection rinse alcohol and dry. Break the bag of the raw material plasma and transfer it to the slurry tank, circulate the water for injection with an interlayer temperature of 30°C to melt the plasma, and perform continuous separation and centrifugation at a speed of 5000-5600 rpm. kg and supernatant.
[0139] (3) Cryoprecipitate dissolution: Cut 18.8kg of cryoprecipitate obtained in step (2) into pieces, diss...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com