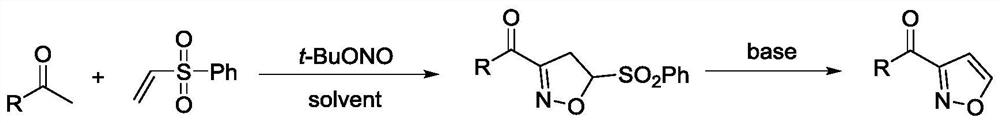
A kind of 3-acyl isoxazole compound and preparation method thereof
A technology for acyl isoxazole and ketone compounds, which is applied in the field of 3-acyl isoxazole compounds and their preparation, and can solve the problems of 3-acyl isoxazole compounds, high toxicity of reaction solvents, and large limitations of ketone types and other problems, to achieve the effects of cheap and easy-to-obtain reaction raw materials and solvents, good prospects for large-scale synthesis, high selectivity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
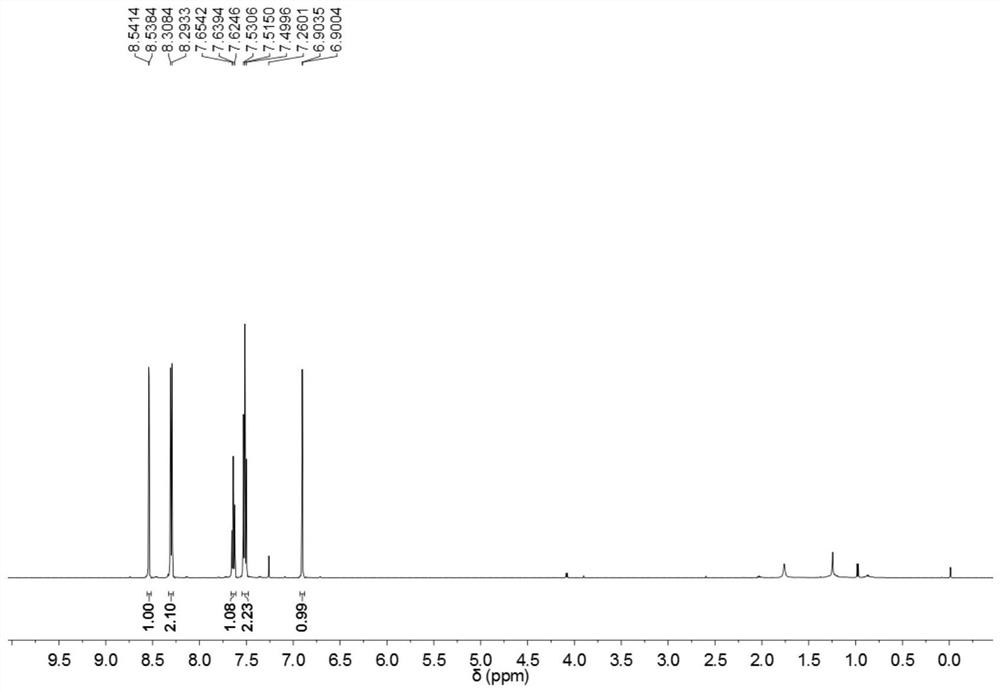
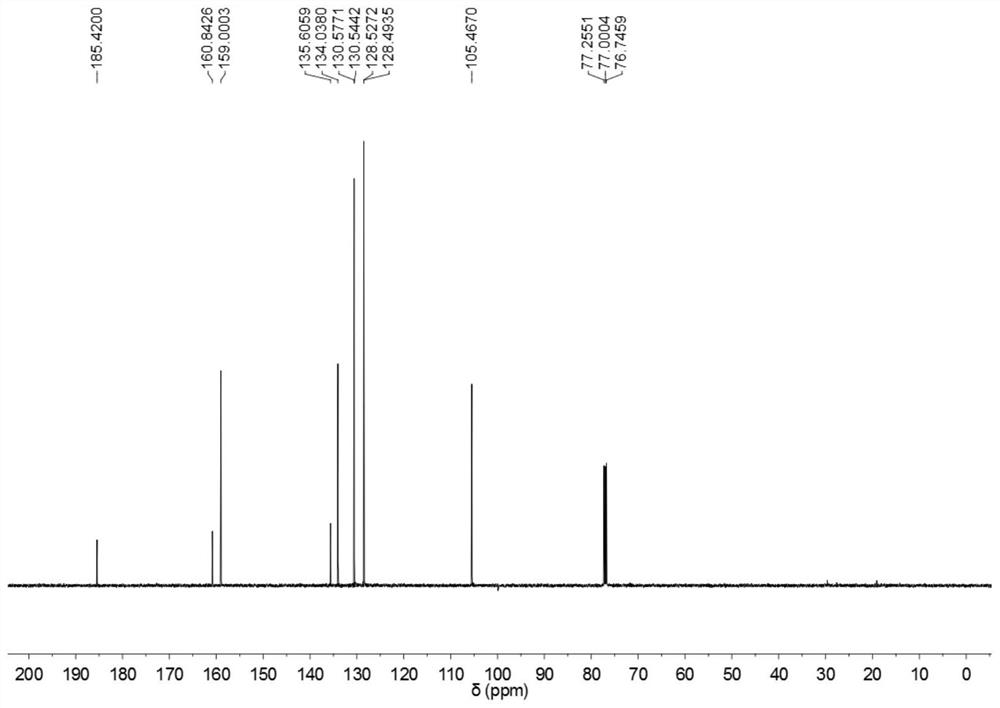
[0032] According to the method of the present invention, 3-isoxazolyl benzophenone is prepared, and the reaction formula is shown in the appendix figure 1 , the specific operation is: in a single-necked flask equipped with acetophenone (0.5mmol) and phenyl vinyl sulfone (1.0mmol), add water (5mL), PTS (2wt%) and tert-butyl nitrite (0.5mmol) ), stirred at a reaction temperature of 25 ° C for 12 hours; continued to add NaOH (5 mol%), continued to stir at a reaction temperature of 140 ° C for 6 hours; after the reaction, washed with water or saturated salt solution, and then extracted with an organic solvent, It was dried, concentrated under reduced pressure to remove the solvent, and the crude product was separated by column chromatography to obtain 3-isoxazolyl benzophenone with a yield of 71%; the obtained 3-isoxazolyl benzophenone is shown in the attached H NMR spectrum. figure 2 , C NMR spectrum, see attached image 3 , H NMR data and C NMR data are as follows:
[0033] ...
Embodiment 2
[0037] According to the method of the present invention, 3-isoxazolyl-p-methylbenzophenone is prepared, and the reaction formula is shown in the appendix figure 1, the specific operation is: in the single-necked flask equipped with p-methylacetophenone (0.5mmol) and phenyl vinyl sulfone (1.5mmol), add water (5mL), Tween-80 (3wt%) and tertiary nitrous acid Butyl ester (1.0 mmol) was stirred at a reaction temperature of 60°C for 10 hours; KOH (5 mol%) was continued to be added, and stirring was continued at a reaction temperature of 130°C for 6 hours; after the reaction, washed with water or saturated salt solution, and then Extracted with organic solvent, dried, concentrated under reduced pressure to remove the solvent, and the crude product was separated by column chromatography to obtain 3-isoxazolyl-p-methylbenzophenone with a yield of 82%.
Embodiment 3
[0039] According to the method of the present invention, 3-isoxazolyl trifluoromethyl benzophenone is prepared, and the reaction formula is shown in the appendix figure 1 , the specific operation is: in the single-necked flask equipped with p-trifluoromethyl acetophenone (0.5mmol) and phenyl vinyl sulfone (2.0mmol), add water (5mL), Triton-X-100 (4wt%) and tert-butyl nitrite (1.5mmol), stirred at a reaction temperature of 80 ° C for 8 hours; continue to add K 2 CO 3 (10mol%), continue to stir for 6 hours at a reaction temperature of 120 ° C; after the reaction, wash with water or saturated salt solution, then extract with an organic solvent, dry, concentrate under reduced pressure to remove the solvent, and the crude product is separated by column chromatography to obtain 3-Isoxazolyltrifluoromethylbenzophenone, 85% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com