Method for synchronously determining angiotensin I and aldosterone in sample
A technology for angiotensin and aldosterone, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of large fluctuation range, poor specificity, affecting the sensitivity and specificity of clinical diagnosis, etc., and achieve high sensitivity, high specificity, high The effect of accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
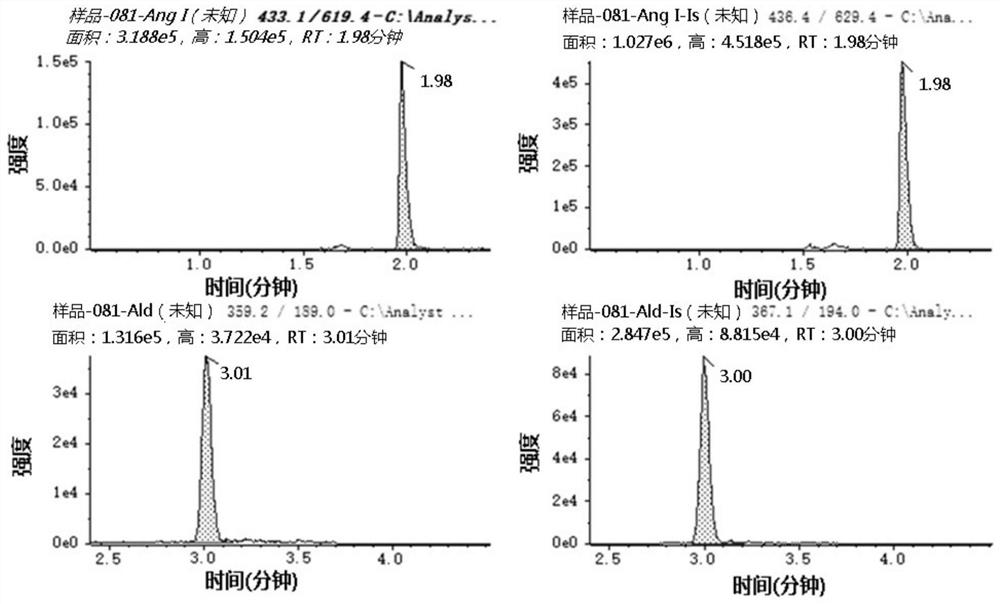
[0229] The detection of embodiment 1Ang I and Ald
[0230] (1) Reagent preparation
[0231] The internal standard adopted in the present embodiment is as follows: Aldosterone: Alderstone-D8, purchased from Cambridge Isotope Laboratories (Cambridge, MA, USA); Angiotensin I: Ang I-[13C6, 15N4], purchased from Cambridge Isotope Laboratories (Cambridge, MA , USA).
[0232] Preparation of standard song and internal standard stock solution : The standard substance of Ang I and the internal standard (Ang I-[13C6, 15N4]) standard substance were dissolved in deionized water respectively to obtain the standard substance stock solution and the internal standard stock solution of angiotensin I. Aldosterone standard and internal standard (Alderstone-D8) standard were dissolved in methanol to obtain aldosterone standard stock and internal standard stock solution. The stock solution is generally at the mg / mL level, such as 2mg / mL. Store the stock solution at -80°C after aliquoting.
[...
Embodiment 2
[0270] Embodiment 2: The method of the present invention is used for the diagnosis of hyperaldosteronism
[0271] (1) The patient is male. The patient complained of fatigue, unsteady walking, palpitations, headache, and cold sweat. The outpatient diagnosis was hypokalemia, hypertension, space-occupying lesions in the right kidney, and suspected primary aldosteronism. However, secondary hypertension due to primary aldosteronism was excluded as judged by confirmatory testing. The patient was eventually diagnosed with hypokalemia (renal potassium loss), bilateral renal cysts, and essential hypertension.
[0272] Take the patient's blood sample (plasma), and use the simultaneous measurement scheme described in the present invention for detection. The ratio ARR results of aldosterone and renin activity measured by sampling at different time points were all less than 30. An example of one of the measurement results is: aldosterone: 6.24ng / dL (aldosterone concentration is finally...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com