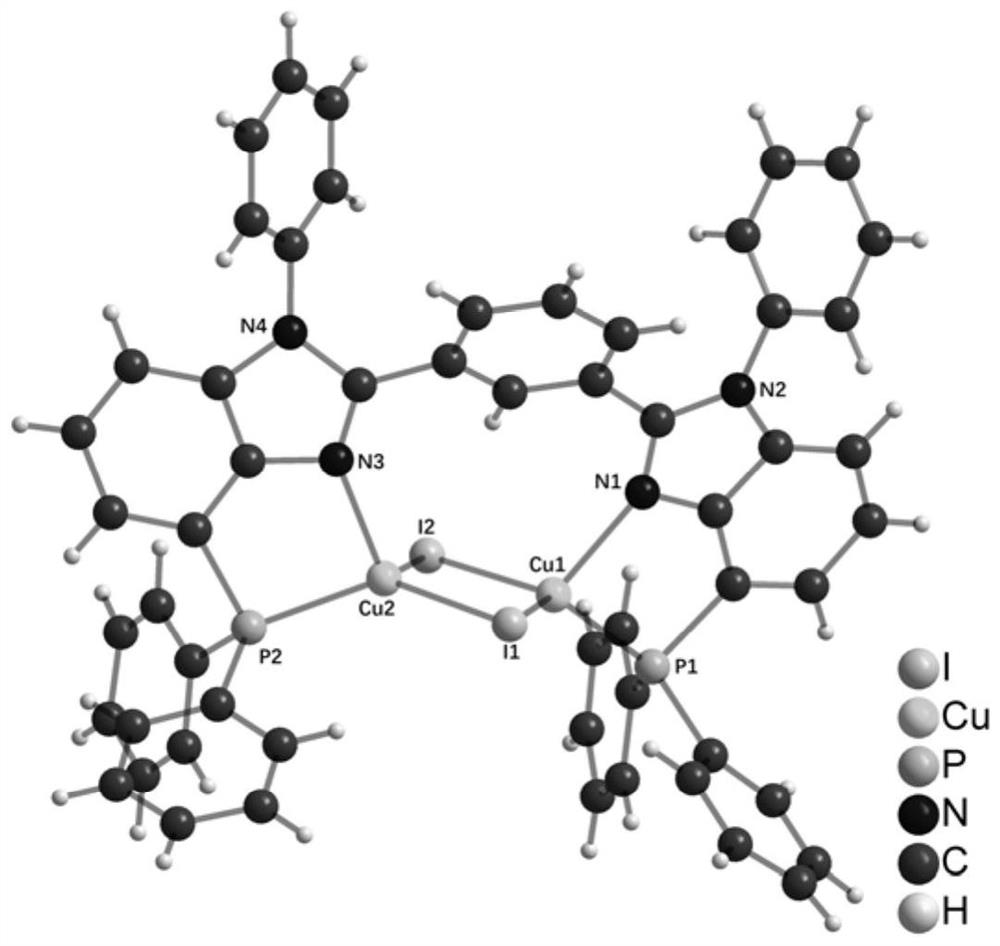
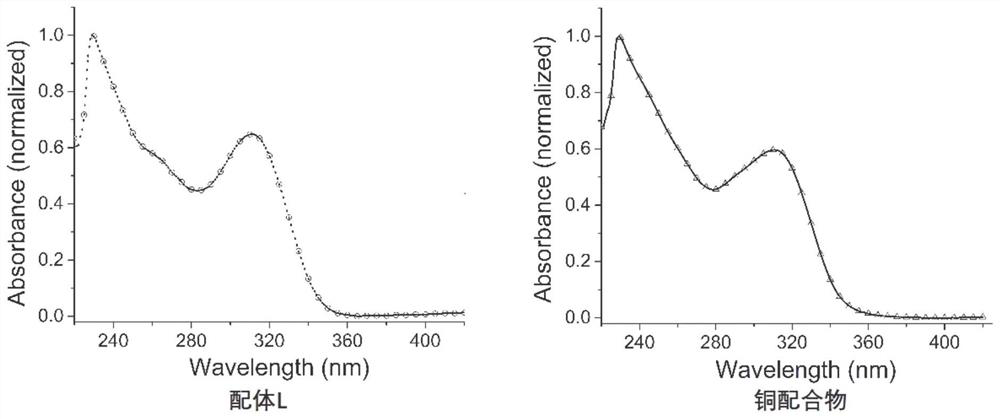
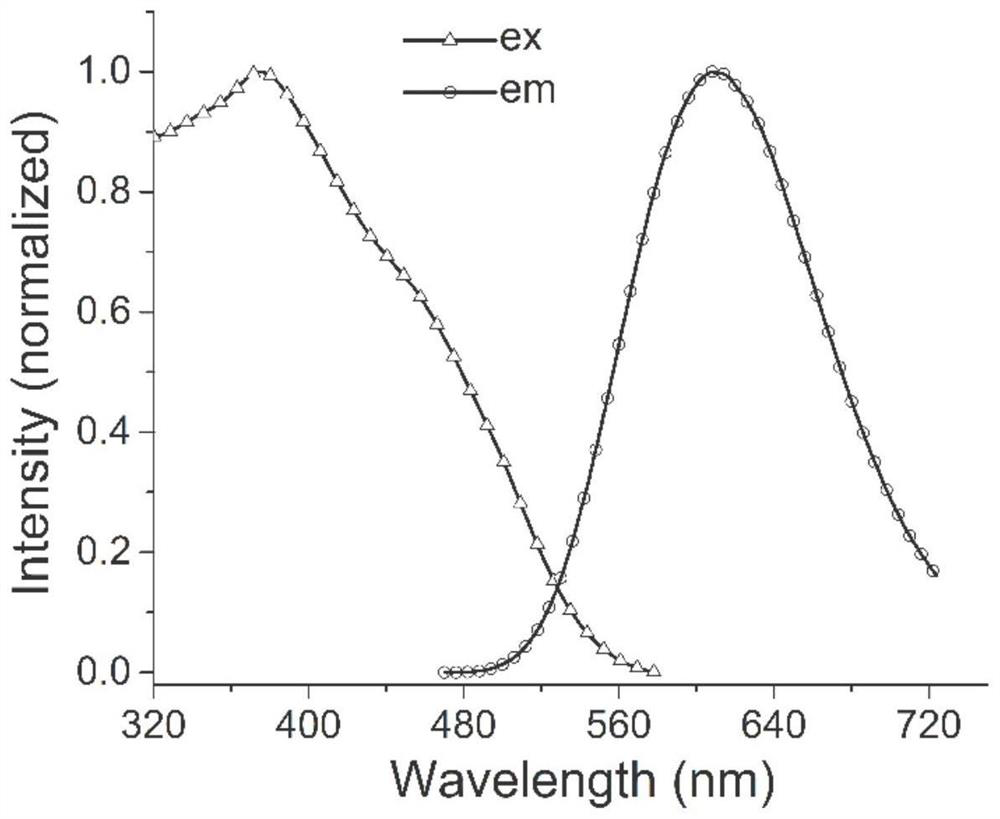
Benzimidazole diphosphine Cu (I) complex and preparation method thereof
A technology of benzimidazole and complex, applied in the field of organic complex synthesis, can solve the problems of hindering application, high price, not abundant pollution, etc., and achieves the effect of good photoluminescence properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051](1) Add aniline (3.3mL, 36.2mmol), 2,6-difluoronitrobenzene (3mL, 30mmol) and anhydrous potassium fluoride (1.8g, 30mmol) into 9mL dimethyl sulfoxide, stir After the dissolution is complete, react at 95°C for 15 hours; after the reaction is completed, cool the system to room temperature, then add 30 mL of distilled water to the reaction system, stir until a large amount of red precipitate appears, filter the precipitate and wash 3 times with 30 ml (10 ml×3 times) of distilled water, The solid was filtered out and dried in a drying oven to obtain intermediate product A;
[0052] (2) Intermediate product A (7g, 20mmol) and m-phthalaldehyde (1.34g, 10mmol) obtained in step (1) are added in 120ml mixed solvent (volume ratio of ethanol and water is 5:1), stir Dissolve, then add sodium dithionite (20g, 120mmol), reflux at 125°C for 8h; after the reaction is complete, cool the system to room temperature, recover the ethanol in the system by rotary evaporation, then extract with...
Embodiment 2
[0056] (1) Add aniline (3.3mL, 36.2mmol), 2,6-difluoronitrobenzene (3mL, 30mmol) and anhydrous potassium fluoride (1.8g, 30mmol) into 9mL dimethyl sulfoxide, stir After the dissolution is complete, react at 100°C for 12 hours; after the reaction is completed, cool the system to room temperature, then add 30 mL of distilled water to the reaction system, stir until a large amount of red precipitate appears, filter the precipitate and wash 3 times with 30 ml (10 ml×3 times) of distilled water, The solid was filtered out and dried in a drying oven to obtain intermediate product A;
[0057] (2) Intermediate product A (7g, 20mmol) and m-phthalaldehyde (1.34g, 10mmol) obtained in step (1) are added in 120ml mixed solvent (volume ratio of ethanol and water is 5:1), stir Dissolve, then add sodium dithionite (20g, 120mmol), reflux at 130°C for 6h; after the reaction is complete, cool the system to room temperature, recover the ethanol in the system by rotary evaporation, then extract wi...
Embodiment 3
[0061] (1) Add aniline (3.3mL, 36.2mmol), 2,6-difluoronitrobenzene (3mL, 30mmol) and anhydrous potassium fluoride (1.8g, 30mmol) into 9mL dimethyl sulfoxide, stir After the dissolution is complete, react at 98°C for 14 hours; after the reaction is completed, cool the system to room temperature, then add 30 mL of distilled water to the reaction system, stir until a large amount of red precipitate appears, filter the precipitate and wash 3 times with 30 ml (10 ml×3 times) of distilled water, The solid was filtered out and dried in a drying oven to obtain intermediate product A;
[0062] (2) Intermediate product A (7g, 20mmol) and m-phthalaldehyde (1.34g, 10mmol) obtained in step (1) are added in 120ml mixed solvent (volume ratio of ethanol and water is 5:1), stir Dissolved, then added sodium dithionite (20g, 120mmol), and refluxed at 128°C for 7h; after the reaction was completed, the system was cooled to room temperature, and the ethanol in the system was recovered by rotary ev...
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescence lifetime | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com