Application of deprotonated phenyl bridged beta-ketimine lithium complex in cyanosilylation reaction
A technology of lithium ketimide and phenyl bridge, applied in lithium complexes and in the field of organic synthesis, can solve the problems such as no reports, and achieve the effects of easy synthesis, mild temperature and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example
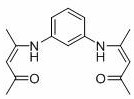
[0027] o-Phenyl bridged β-ketoimine ligand (L ph h 2 )Synthesis
[0028]
[0029] Add 150 ml of absolute ethanol, 10.8 g of m-phenylenediamine (100 mmol), 20.5 mL of acetylacetone (200 mmol), and a catalytic amount of p-toluenesulfonic acid into a three-necked flask, heat and reflux for 24 hours to obtain a reddish-brown liquid and pale The yellow solid mixture was suction filtered, and the solid was recrystallized from absolute ethanol to obtain 24.5 g of light yellow needle-like crystals, with a yield of 90%, which was Ligand L ph h 2 . 1 H NMR (400 MHz, CDCl 3 ): δ12.47 (2H, s, N H ), 7.32-7.27 (1H, m, Ar H ), 6.94-6.91 (2H, m, Ar H ), 6.86 (1H, s, Ar H ), 5.21 (2H, s, C H =C(CH 3 )N), 2.10 (6H, s, C H 3 ), 2.01 (6H,s,C H 3 ). 13 C NMR (101 MHz, CDCl 3 ): δ 196.54 ( C OCH 3 ), 159.62 ( C =CH), 139.63(Ar C ), 129.71 (Ar C ), 121.45 (Ar C ), 120.43 (Ar C ), 98.20 (= C H), 29.25 ( C h 3),19.94 ( C h 3 ). HRMS (ESI-MS) calcd. for C 16 h 20 N 2...
Embodiment 1
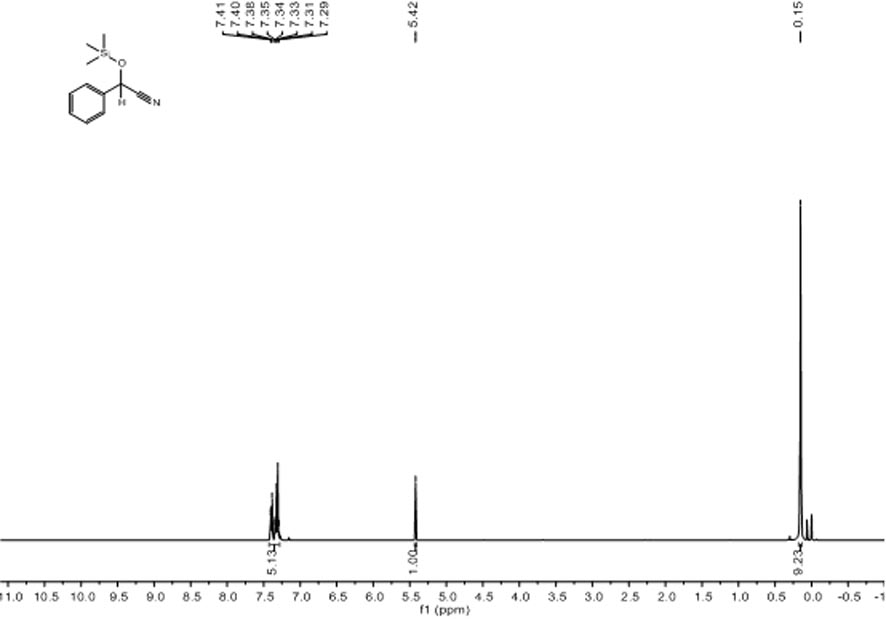
[0039] Embodiment one: [L ph’ Li 4 (THF) 4 ] 2 Catalytic Reduction of Benzaldehyde and TMSCN
[0040] Under nitrogen atmosphere, add catalyst 0.6 mg (0.0005 mmol, 0.05%) to the reaction flask after dehydration and deoxygenation treatment, add benzaldehyde (101.6 μL, 1.0 mmol), TMSCN (137.6 μL, 1.1 mmol) with a pipette gun ), after reacting at room temperature for 15 min, use a dropper to draw a drop into the NMR tube, add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 99%. NMR data of the product: 1 H NMR (400 MHz, CDCl 3 ) δ7.41-7.29 (m, 5H, Ar H ),5.42 (s, 1H, C H OSi(CH 3 ) 3 ), 0.15 (s, 9H, Si(C H 3 ) 3 ), see the map figure 1 .
Embodiment 2
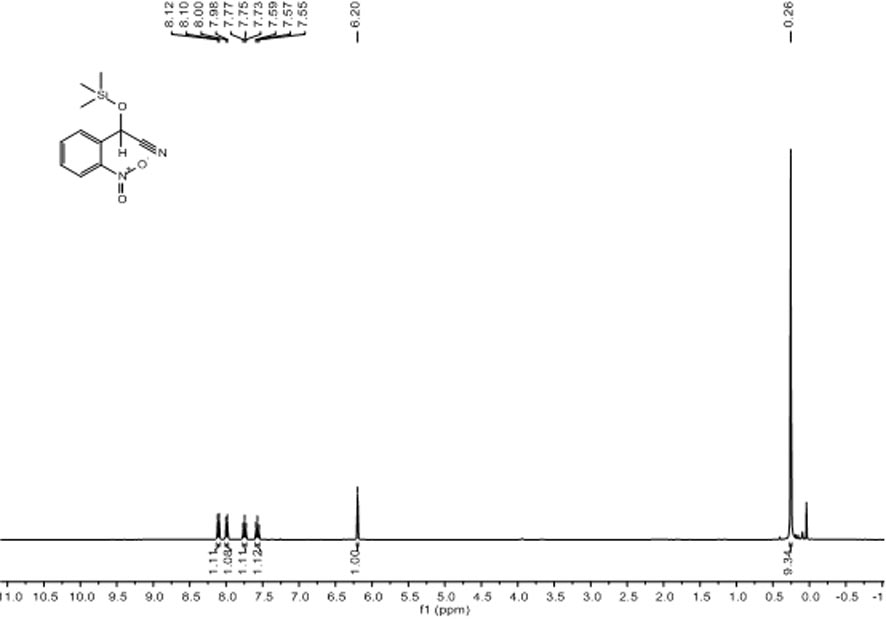
[0041] Embodiment two: [L ph’ Li 4 (THF) 4 ] 2 Catalytic Reduction of p-Fluorobenzaldehyde and TMSCN
[0042] Under nitrogen atmosphere, add catalyst 0.6 mg (0.0005 mmol) to the reaction flask after dehydration and deoxygenation treatment, add p-fluorobenzaldehyde (107.2 μL, 1.0 mmol) and TMSCN (137.6 μL, 1.1 mmol) sequentially with a pipette gun , after reacting at room temperature for 15 min, use a dropper to draw a drop into the nuclear magnetic tube, add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 99%. NMR data of the product: 1 H NMR (400 MHz, CDCl 3 ) δ7.48-7.44 (m, 2H, Ar H ), 7.12-7.08(m, 2H, Ar H ), 5.48 (s, 1H, C H OSi(CH 3 ) 3 ), 0.23 (s, 9H, Si(C H 3 ) 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com