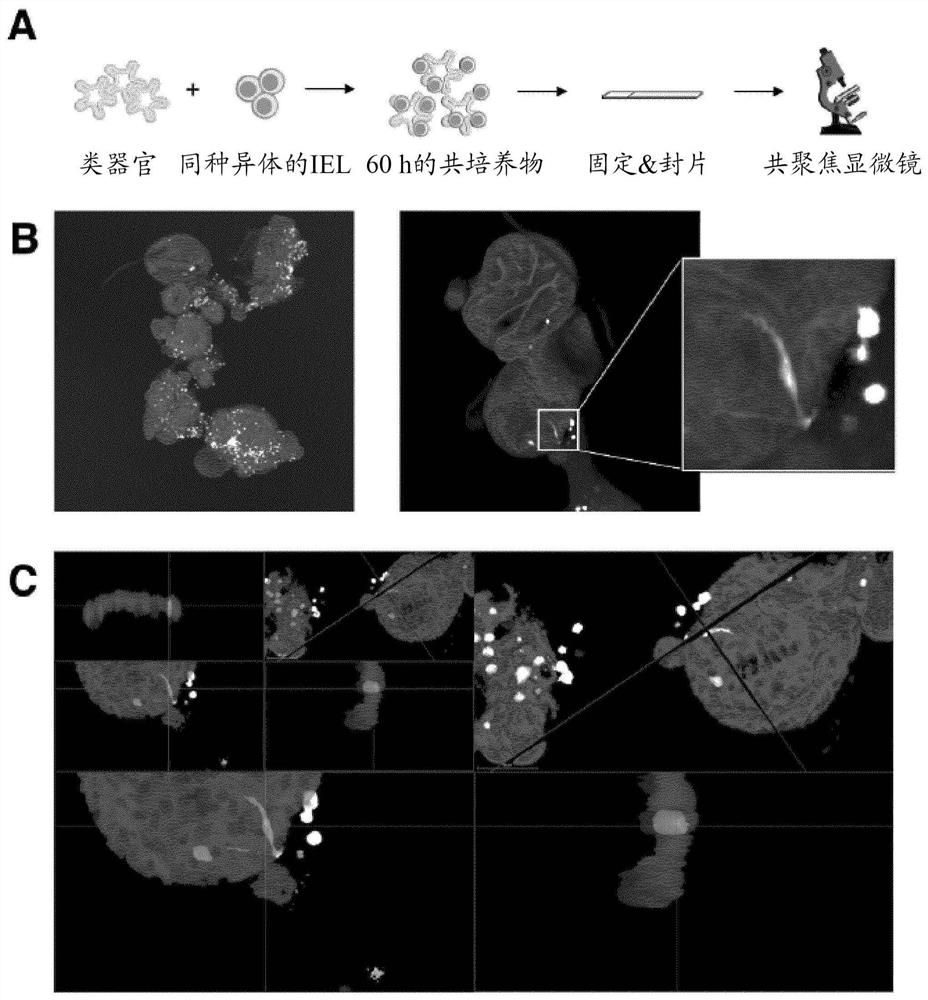
Immune cell organoid co-cultures
A technology of co-culture and immune cells, applied in the field of co-culture of immune cell organoids, can solve the problems of immune cell co-culture cancer treatment plan, no report of human organoid co-culture progress cancer application and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
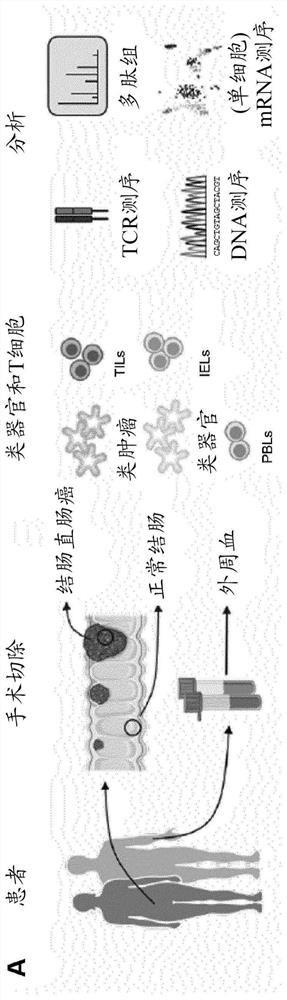
[0273] Example 1. Collection of normal colon and colorectal cancer biopsies from a hospital.
[0274] This example shows the isolation of cell samples, which are used in subsequent examples to prepare organoid, tumor and immune cell samples.
[0275] Biopsies of normal colonic mucosa and tumor tissue were obtained from the resected colon and / or rectum of patients with colorectal cancer. Peripheral blood was also drawn during surgery.
[0276] Specifically, biopsy tissues from human colorectal cancer tissues as well as normal (adult) human colonic mucosal epithelium were collected in 50 mL standard tubes containing ice-cold 10-15 mL of high-grade DMEM / F12 medium completely With penicillin / streptomycin (from 100× stock solution of 10,000 U / mL penicillin and 10K μM / mL streptomycin), HEPES (from 100× stock solution of 1M), GlutaMAX (from 100× stock solution; all Gibco TM ) and the Rho kinase inhibitor Y-27632 (Sigma-Aldrich). Biopsies were kept on ice and processed immediately,...
Embodiment 2
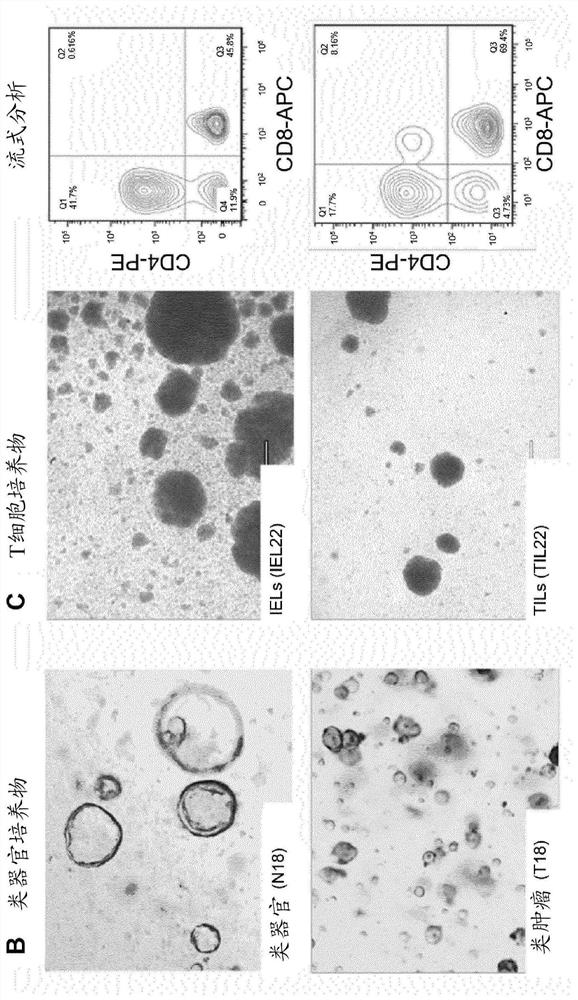
[0278] Example 2. Isolation of crypts from normal colon tissue and derivation of normal colon organoids; isolation of intraepithelial T cells from normal colon tissue for T cell culture.
[0279] This example shows the processing of normal colon samples for the development of organoid cultures and for the isolation of immune cells from normal colon samples.
[0280] Normal colonic mucosa was treated with EDTA to release crypts for deriving normal colonic organoids, and then further digested to prepare single-cell suspensions containing intraepithelial lymphocytes (IELs) for T cell culture.
[0281] Isolation of crypts and derivation of normal colon organoids from normal colon tissue.
[0282] Remove the muscle layer and fat under a dissecting microscope using surgical scissors and forceps. Cut the cleaned tissue into thin strips of approximately 1-2 mm. One strip was fixed in 4% formaldehyde (Sigma-Aldrich) for histological analysis, and one strip was snap frozen (in dry ice...
Embodiment 3
[0286] Example 3. Digestion of colorectal cancer tissue for tumor organoid and T cell culture; derivation of colorectal cancer tumoroids.
[0287] This example shows the processing of cancerous colon samples for the development of tumor-like cultures, and the isolation of immune cells from cancerous colon samples.
[0288] Tumor tissue was digested to prepare single-cell suspensions containing epithelial tumor cells, tumor-infiltrating lymphocytes (TILs) for deriving tumoroids, and for T-cell culture.
[0289] Digestion of colorectal cancer tissue for tumor and T cell culture.
[0290] Tumor biopsies were cut into thin strips of approximately 1-2 mm. One strip was fixed in 4% formaldehyde for histological analysis, and each strip was snap frozen (in dry ice or liquid nitrogen) and stored at -80°C for genetic and / or protein analysis. The remaining strips were further cut using forceps until the tumor mass appeared viscous. Tumor masses were incubated in 10 mL of complete hig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com