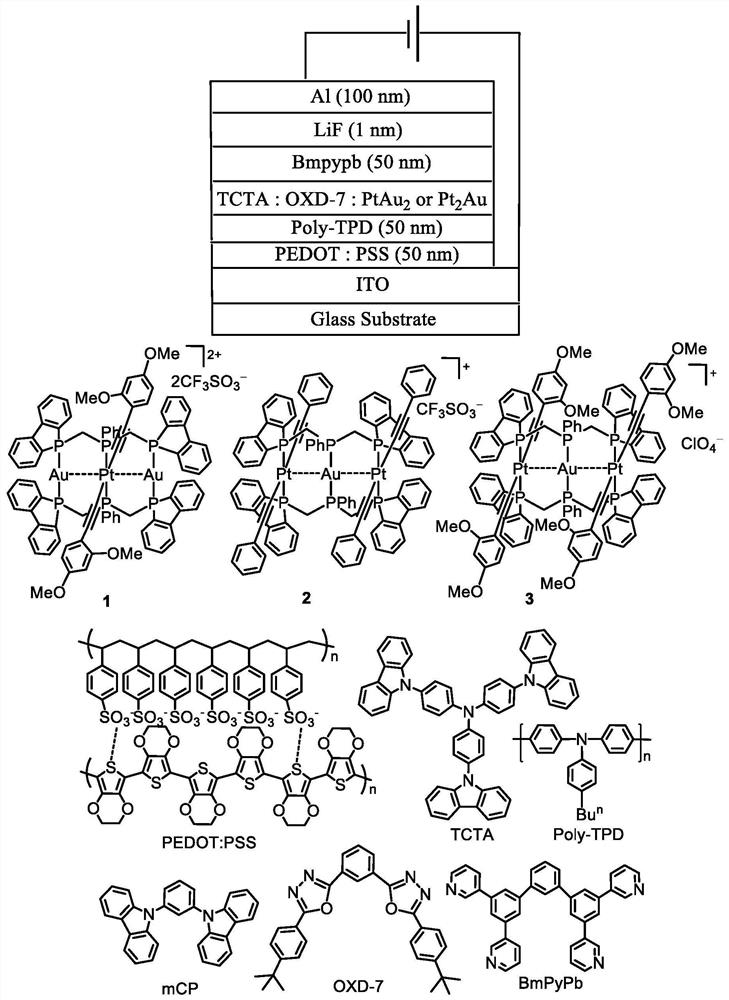
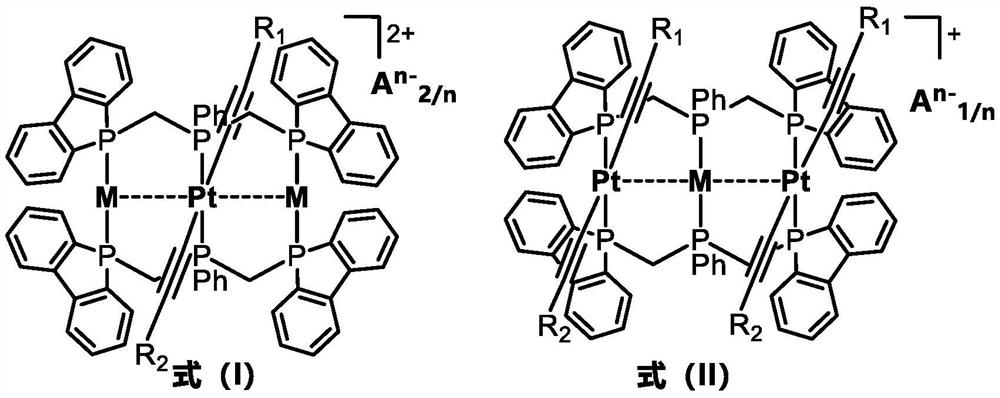
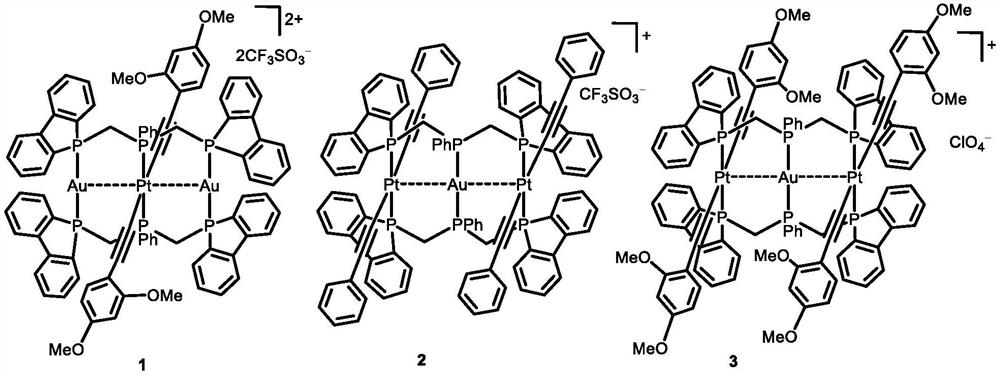
Rigid triphosphine-supported phosphorescent Pt-M complex and organic light emitting diode thereof
A technology of light-emitting diodes and complexes, applied in the fields of light-emitting materials, organic chemistry, platinum group organic compounds, etc., can solve the problems of lack of exploration, limited research on phosphorescent multi-nuclear complexes, etc., and achieve the effect of strong phosphorescence emission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1: Complex [PtAu 2 (dBpimp) 2 (C≡CC 6 h 3 (OMe) 2 -2,4) 2 ](CF 3 SO 3 ) 2 (1) Preparation
[0058] [Au(tht) was dissolved in 20mL 2 ](CF 3 SO 3 ) (0.1 mmol) in dichloromethane solution was added dBpimp (0.1 mmol). After stirring for 30 minutes, Pt(PPh 3 ) 2 (C≡CC 6 h 3 (OMe) 2 -2,4) 2 (0.05 mmol) in dichloromethane (5 mL), and the reaction solution was stirred at room temperature for 4 hours. The product was purified by silica gel column chromatography using CH 2 Cl 2 -MeCN (8:1) was collected as eluent to give pure product. Yield: 26%. High resolution mass spectrometry [M-2CF 3 SO 3 ] 2+ Calculated value: 958.1268, Measured value: 958.1256. H NMR spectrum (CD 2 Cl 2 ,ppm):8.78-8.75(m,4H),8.15-8.10(m,4H),7.98-7.90(m,10H),7.78-7.74(t,4H),7.68-7.64(t,4H),7.55 -7.51(m,4H),7.39-7.33(m,6H),7.02-7.00(m,8H),6.75-6.73(m,2H),6.52-6.51(d,2H),4.96-4.89(m, 4H), 4.00-3.85(m, 10H), 3.63(s, 6H). Phosphorus NMR spectrum (CD 2 Cl 2 ,ppm):20.28(...
Embodiment 2
[0059] Embodiment 2: Complex [Pt 2 Au(dBpimp) 2 (C≡CC 6 h 5 ) 4 ](CF 3 SO 3 ) (2) preparation.
[0060] [Au(tht) was dissolved in 20mL 2 ](CF 3 SO 3 ) (0.05mmol) in dichloromethane solution was added dBpimp (0.1mmol). After stirring for 30 minutes, Pt(PPh 3 ) 2 (C≡CC 6 h 6 ) 2 (0.1 mmol) in dichloromethane (5 mL). The reaction solution turned orange after being stirred at room temperature for 4 hours. The product was purified by silica gel column chromatography using CH 2 Cl 2 -MeCN(8:1) was the eluent to collect the orange product. Yield: 80%. High resolution mass spectrometry [M-ClO 4 ] + Calculated value: 1996.2882, Measured value: 1996.2880. H NMR spectrum (CD 2 Cl 2 ,ppm):8.82-8.79(m,4H),8.16-8.14(m,4H),7.88-7.81(m,8H),7.72-7.69(t,2H),7.63-7.59(t,4H),7.51 -7.35(m,16H),7.31-27(m,2H),7.21-7.13(m,14H),6.85-6.81(m,4H),6.62-6.60(m,4H),4.32-4.25and 3.52- 3.48(m,8H). Phosphorus NMR spectrum (CD 2 Cl 2 ,ppm):-2.95(m,2P,J Pt–P =2618Hz,J P–P =26.78Hz)...
Embodiment 3
[0061] Embodiment 3: Complex [Pt 2 Au(dBpimp) 2 (C≡CC 6 h 3 (OMe) 2 -2,4) 4 ](ClO 4 ) (3) preparation.
[0062] [Au(tht) was dissolved in 20mL 2 ]ClO 4 (0.05 mmol) in dichloromethane was added dBpimp (0.1 mmol). After stirring for 30 minutes, Pt(PPh 3 ) 2 (C≡CC 6 h 3 (OMe) 2 -2,4) 2 (0.1 mmol) in dichloromethane (5 mL). The reaction solution turned orange after being stirred at room temperature for 4 hours. The product was purified by silica gel column chromatography using CH 2 Cl 2 -MeCN(8:1) was the eluent to collect the orange product. Yield: 80%. High resolution mass spectrometry [M-ClO 4 ] + Calculated: 2236.3728, Measured: 2236.3727. H NMR spectrum (CD 2 Cl 2 ,ppm):8.81-8.78(m,4H),8.07-8.06(m,4H),7.86-7.78(m,8H),7.57-7.53(m,6H),7.43-7.31(m,16H),7.06 -7.04(d,2H),6.78-6.75(m,4H),6.66-6.64(d,2H),6.46-6.45(d,2H),6.35-6.33(m,2H),6.22(br,2H) , 6.17-6.14 (m, 2H), 4.51-4.48 (m, 8H), 3.91-3.82 (m, 12H), 3.56-3.42 (m, 16H). Phosphorus NMR spectrum (CD 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com