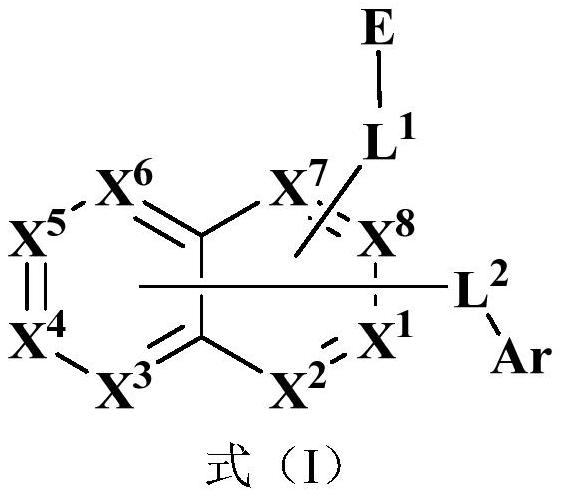
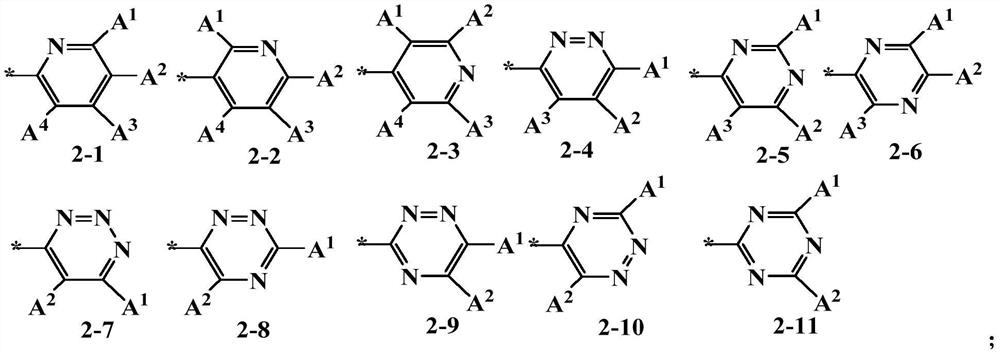
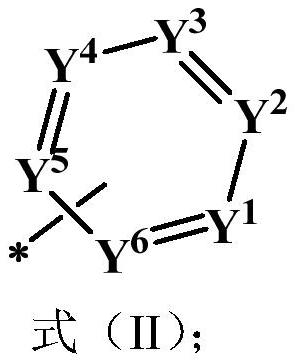Compound, application thereof and organic electroluminescence device containing same
A compound, independent technology, applied in the field of organic electroluminescent devices to achieve the effect of strong electron deficiency, high electron injection ability, and improved electron injection and migration efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0105] Preparation of Compound C5
[0106]
[0107] (1) Preparation of compound 1-1:
[0108] 2-Chloro-3-quinolineboronic acid (20.7g, 100mmol), 3-bromo[1,1'-biphenyl]-3-cyanide (25.7g, 100mmol), potassium carbonate (41.4g, 300mmol), four (Triphenylphosphine)palladium (1.15g, 1mmol) was added into a flask containing 500mL of toluene, 100mL of ethanol and 100mL of water, heated under reflux under a nitrogen atmosphere for 4 hours, and TLC showed that the reaction was complete. Cool to room temperature, separate the liquids, extract the aqueous phase with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, filter, spin dry under reduced pressure to obtain a crude product, separate and purify by column chromatography to obtain compound 1-1 (29.6g, 87% ).
[0109] (2) Preparation of compound 1-2
[0110] Compound 1-1 (27.2g, 0.08mol), boric acid pinacol ester (30.5g, 0.12mol) and potassium acetate (123.5g, 0.24mol) were added to 1,4-dioxane (0.5L) ...
preparation example 2
[0114] Preparation of Compound C39
[0115]
[0116] (1) Preparation of compound 2-1:
[0117] 3-bromo-4-chloroquinoline (24.1g, 100mmol), 2-(3-boronate phenyl)-4,6-diphenyl-1,3,5-triazine (43.5g, 100mmol ), salt of wormwood (41.4g, 300mmol), pd (PPh3) 4 (1.15g, 1mmol) add and contain 500mL toluene, in the flask of 100mL ethanol and 100mL water, nitrogen atmosphere heating reflux reaction 4 hours, TLC shows that reaction is complete. Cool to room temperature, separate the liquids, extract the aqueous phase with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, filter, spin dry under reduced pressure to obtain a crude product, separate and purify by column chromatography to obtain compound 2-1 (40g, 85%) .
[0118] (2) Preparation of compound 2-2
[0119] Compound 2-1 (37.6g, 0.08mol), boric acid pinacol ester (30.5g, 0.12mol) and potassium acetate (123.5g, 0.24mol) were added to 1,4-dioxane (0.5L) In the flask, palladium acetate (180mg, 0.8mm...
preparation example 3
[0123] Preparation of Compound C46
[0124]
[0125] (1) Preparation of compound 3-1:
[0126] 2-Chloro-6-bromoquinoline (24.1g, 100mmol), 4-cyano [1,1'-biphenyl] boronic acid (22.3g, 100mmol), potassium carbonate (41.4g, 300mmol), four (three Phenylphosphine)palladium (1.15g, 1mmol) was added into a flask containing 500mL of toluene, 100mL of ethanol and 100mL of water, and heated under reflux under a nitrogen atmosphere for 4 hours. TLC showed that the reaction was complete. Cool to room temperature, separate the liquids, extract the aqueous phase with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, filter, and spin dry under reduced pressure to obtain a crude product, which is separated and purified by column chromatography to obtain compound 3-1 (28.9 g, 85% ).
[0127] (2) Preparation of compound 3-2
[0128] Compound 3-1 (27.2g, 0.08mol), boric acid pinacol ester (30.5g, 0.12mol) and potassium acetate (123.5g, 0.24mol) were added to 1,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com