Preparation method of cell for expressing anti-CD22 chimeric antigen receptor and PD-L1 blocking protein, expression vector and application
A technology of chimeric antigen receptor and PD-L1, which is applied in the field of medicine and biology, can solve problems such as failure to achieve success, achieve the effect of prolonging the action time, alleviating immune tolerance, and improving tumor killing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
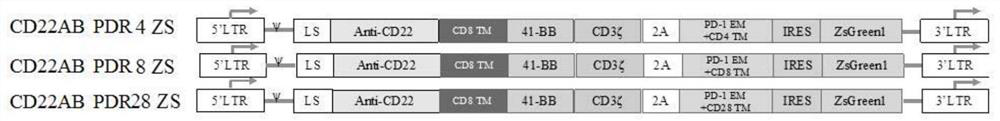
[0067] In this example, a series of anti-CD22 vectors were constructed to co-express CAR and PD-L1 blocking protein. The anti-CD22 CAR constructs used in the present invention are second-generation CARs, all of which contain a CD8α leader sequence, an anti-CD22-specific scFv, a CD8α hinge, and a TM (transmembrane domain). The intracellular domain contains the 4-1BB co-stimulatory domain and the CD3ζ signaling domain, known as CD22AB.
[0068] To identify the efficiency of immune checkpoint molecules in CAR T cells, we constructed vectors co-expressing CAR and PD-1 extracellular domains with different transmembrane regions (CD4, CD8, and CD28). Each vector was designated 22ABPDR 4, 22ABPDR 8 and 22ABPDR28, respectively. Inserted gene and plasmid construction reference figure 1 shown.
[0069] The vector includes the first nucleic acid encoding a chimeric antigen receptor consisting of a single-chain antibody of an antibody against CD22 (Anti-CD22), a CD8 transmembrane domain...
Embodiment 2
[0104] In this example, T cells co-expressing CAR and PDR with CD28 were respectively constructed. Inserted gene and plasmid construction reference image 3 shown. Four kinds of plasmid vectors ZS, PDR, CD22AB and CD22ABPDR were constructed by conventional technical means in the field. Among them, the vector ZS is a control vector containing nucleic acids encoding IRES and ZsGreen1; the vector PDR is a control vector containing nucleic acids encoding PD-1 EM and CD28 TM; the vector CD22AB is a control vector containing nucleic acids encoding Anti-CD22, CD8 TM, 41-BB, CD3ζ, IRES and ZsGreen1 nucleic acid control vector; and the vector CD22ABPDR is the same as the vector CD22ABPDR 28 ZS in Example 1.
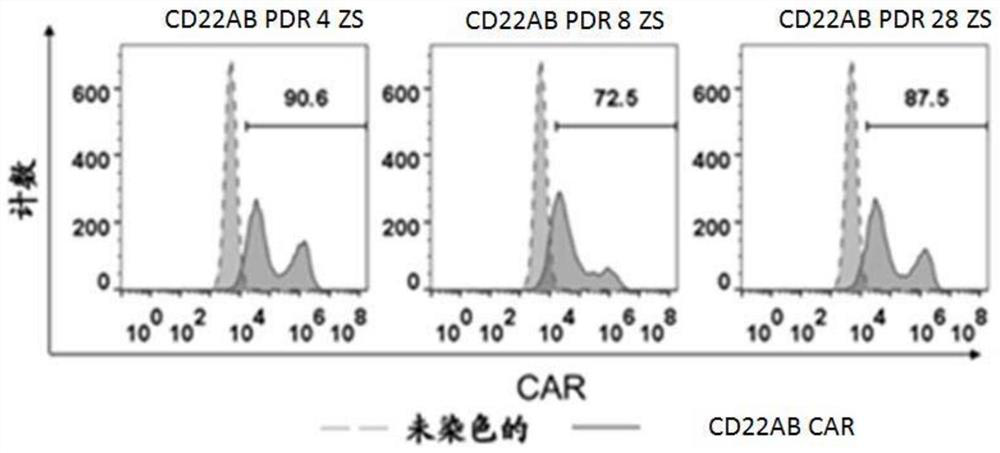
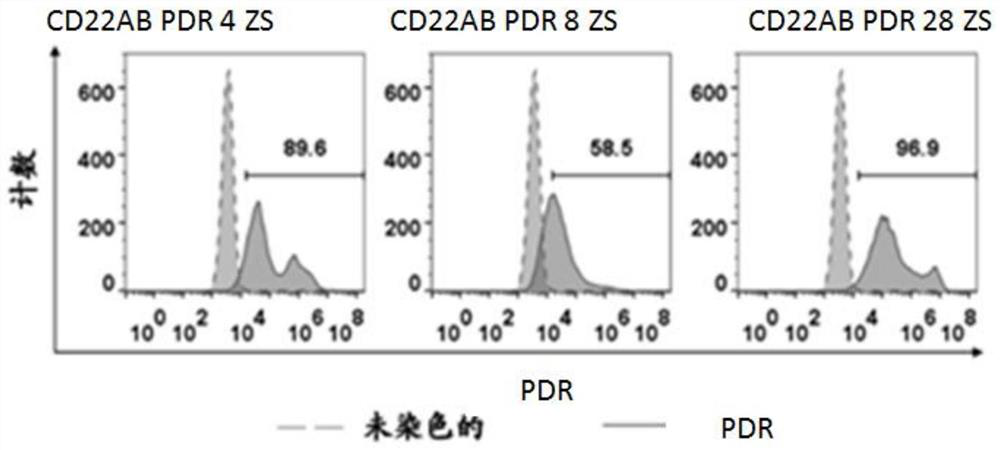
[0105] The above four plasmid vectors were transfected into human peripheral blood lymphocytes to construct CAR T cells.
[0106] The transfected human peripheral blood lymphocytes were cultured and harvested for FACS detection and Q-PCR identification.
[0107] The preparatio...
Embodiment 3
[0113] This example verifies the effect of the constructed CAR T cells.
[0114] (1) Construct a cell line expressing PD-L1.
[0115] To characterize the function of PDRs expressed on CAR T cells, tumor cells need to express PD-L1. Because the tumor cell lines of Raji, K562, Daudi, and BV173 do not express endogenous PD-L1, the inventors used lentivirus to construct PD-L1-expressing tumor cell lines on the basis of Raji, K562, Daudi, and BV173. Tumor cell lines Raji-PD-L1, K562-PD-L1, Daudi-PD-L1 and BV173-PD-L1. Through flow cytometry analysis, it was determined that all constructed cell lines could stably and highly express PD-L1 (refer to Figure 5 shown).
[0116] (2) Expression detection of key activation markers.
[0117] The ratios of CD4 and CD8 and other activated cell markers expressed on human PMBC transfected by the four plasmid vectors ZS, PDR, 971 and 971PDR in Example 2 were analyzed by flow cytometry.
[0118] Depend on Figure 6A It can be seen that the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com