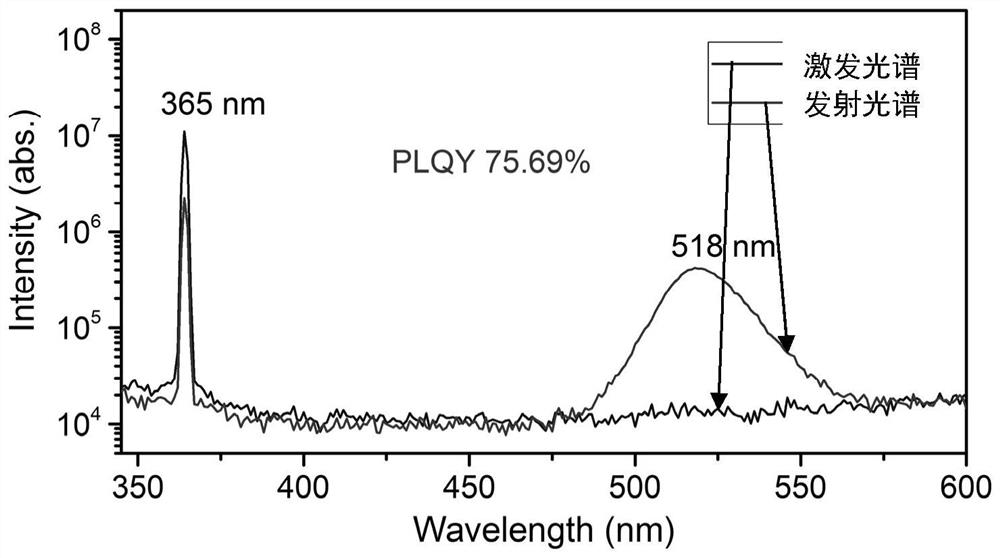
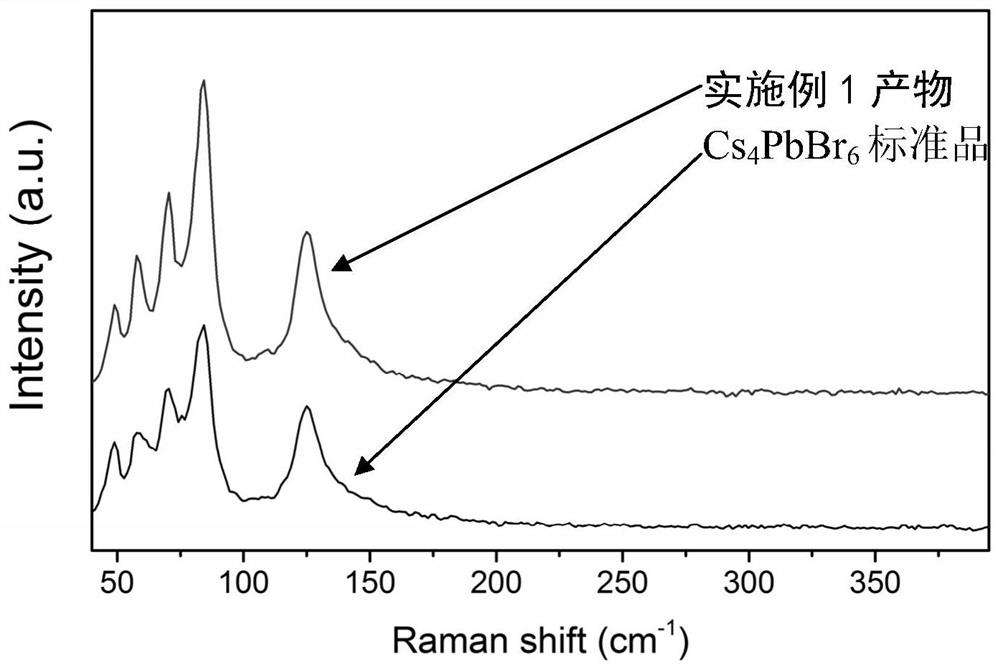
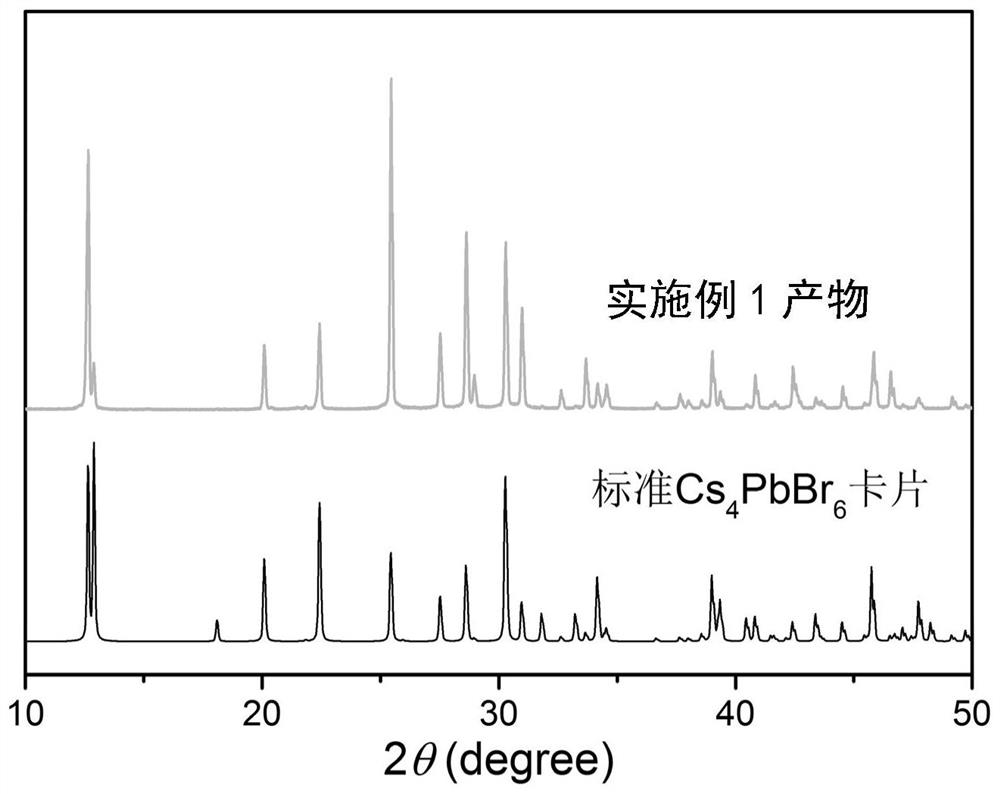
Zero-dimensional cesium-lead-bromine inorganic perovskite material and preparation method thereof
A zero-dimensional cesium lead and inorganic calcium technology, applied in inorganic chemistry, luminescent materials, chemical instruments and methods, etc., can solve the problems of difficulty in large-scale synthesis and high synthesis cost, and achieve mild and controllable preparation conditions, stable properties, and products. high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The first aspect of the embodiment of the present application provides a method for preparing a zero-dimensional cesium lead bromine inorganic perovskite material, comprising the following steps:
[0026] Dissolving the first lead source in a mixed solution of DMF and DMSO to obtain the first solution;
[0027] CsBr is dissolved in water to obtain a second solution;
[0028] After thermal mixing treatment of the first solution and the second solution, cooling treatment and separation treatment, Cs 4 PbBr 6 Inorganic perovskite materials, that is, zero-dimensional cesium lead bromine inorganic perovskite.
[0029] The zero-dimensional cesium lead bromide (Cs) provided by the first aspect of the application 4 PbBr 6 ) The preparation method of the inorganic perovskite material, using a mixed solvent of DMF and DMSO to dissolve the lead source, using water to dissolve CsBr, and after mixing the first solution and the second solution, a three-solvent system of water-DMS...
Embodiment 1
[0051] a Cs 4 PbBr 6 An inorganic perovskite material, the preparation steps of which include:
[0052] 1. Add 10mmol PbBr 2 Dissolve in a mixed solution of 20mL DMF and 10mL DMSO, and heat to 80-90°C to obtain the first solution;
[0053] 2. Take 4mL of 10Mmol / L CsBr aqueous solution and heat it to 90°C. After clarification, the second solution is obtained;
[0054] 3. Mix and stir the first solution with a temperature of 80-90°C and the second solution with a temperature of about 90°C, and naturally cool to room temperature;
[0055] 4. After centrifugation, the obtained solid was washed twice with toluene and n-hexane with a volume ratio of 1:2, put into a vacuum drying oven, dried for more than 8 hours, and stored dry to obtain Cs 4 PbBr 6 Inorganic perovskite materials.
Embodiment 2
[0057] a Cs 4 PbBr 6 A cyclic preparation method for an inorganic perovskite material, the steps comprising:
[0058] 1. In the mother liquor after centrifugation in Example 1, add the PbBr of 8mmol 2 After fully stirring, add 16ml of 2M CsBr aqueous solution;
[0059] 2. After heating the system to 80°C, continue to stir, and evaporate excess water under low pressure. After the system solution changes from orange to yellow-green, stop heating and cool down to room temperature naturally;
[0060] 3. After centrifugation, the obtained solid was washed twice with toluene and n-hexane with a volume ratio of 1:2, put into a vacuum drying oven, dried for more than 8 hours, and dried to obtain Cs 4 PbBr 6 Inorganic perovskite materials.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com