Ischemic retinopathy detection biomarker, detection kit and application
A retinopathy and biomarker technology, applied in the field of biomedical detection, can solve the problem of no circRNA-Wdr37, and achieve the effect of reducing treatment and medical costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
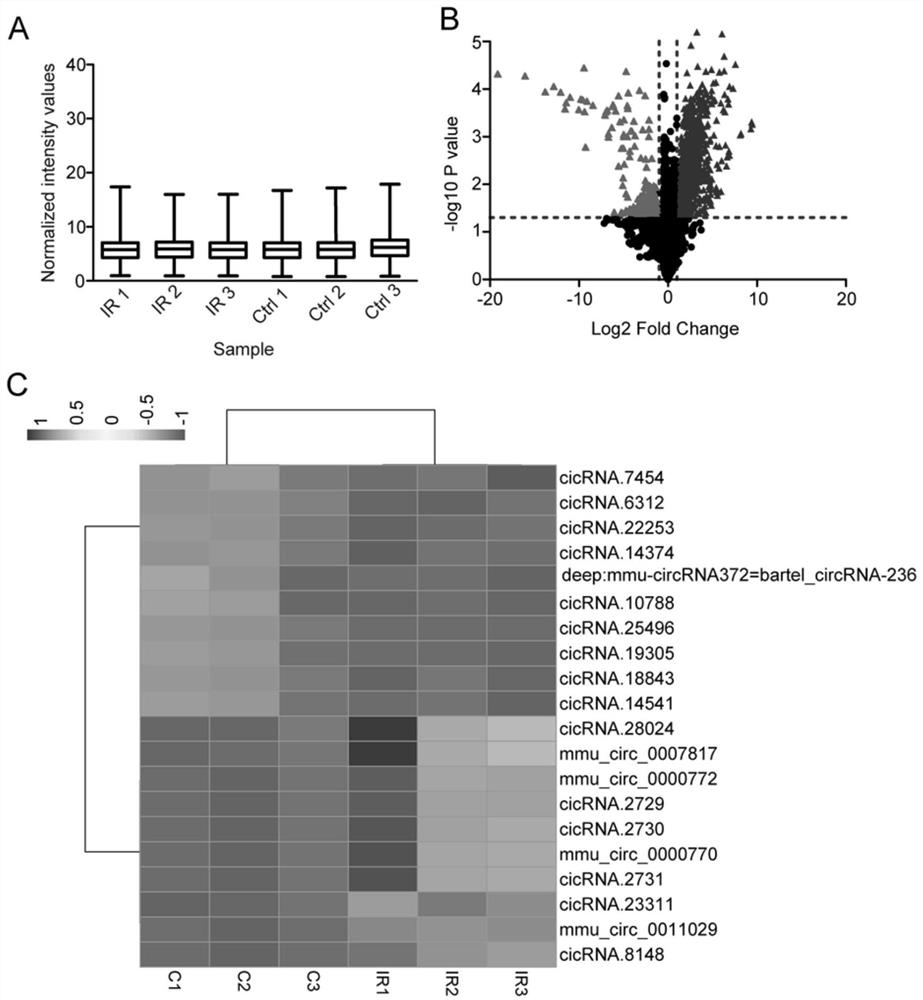
[0036] Example 1 circRNA-Wdr37 ischemic retinopathy validation embodiment
[0037] circRNA microarray screening related circRNA ischemic retinopathy disease and verify it.
[0038] Step: Sample Preparation: Construction of mouse retinal ischemia reperfusion (IR) animal model, taking the experimental group (IR, n = 3) and control (Ctrl, n = 3) mice retinal tissue. RNA was extracted with Trizol (Invitrogen) reagent and preserved at -80 ° C spare.
[0039] Step two: Screening of differentially expressed circRNA:
[0040] American companies circRNA Aglient using cDNA microarray analysis circRNA related ischemic retinopathy; Anal specific steps: use of enzyme labeled fluorophore labeled circRNA, obtained with fluorescent probes for microarray hybridization, the labeling conditions in MAUI hybridization using the apparatus and chip hybridization; fluorescence intensity using GenePix 4000B scanner chip chip, and the experimental result into numerical data is saved; distribution of each s...
Embodiment 2
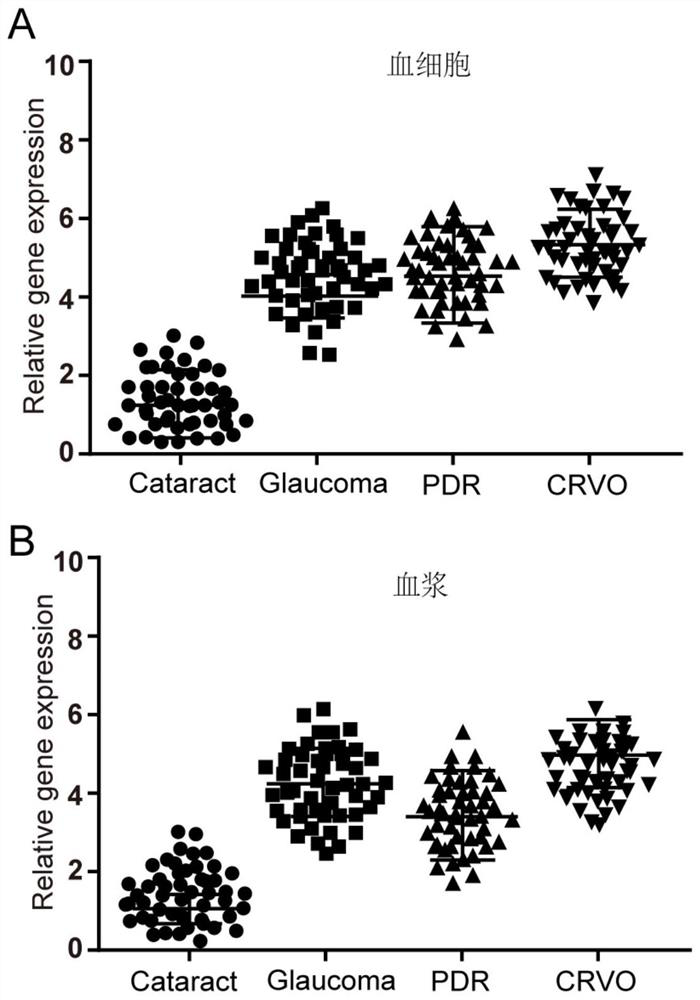
[0045] CircRNA-Wdr37 expression was detected in the blood cells and plasma in Example 2
[0046] The first step: separating serum and plasma samples
[0047] Were collected to obtain the experimental group (primary open-angle glaucoma, central vein occlusion and diabetic retinopathy) and control group (cataract) of each sample of each patient's blood 200 cases, the use of heparin tubes using serum was separated therefrom centrifugally and plasma RNA, for detecting circRNA. Centrifugation conditions of 4 ℃, 12,000rpm, 10min.
[0048] Step two: RNA extraction of serum and plasma samples
[0049] Respectively, in the isolated blood cells and plasma samples after the addition of TRIzol room temperature for 10min, the sample was sufficiently lysed (Note: If the next operation is not performed, a sample may be placed in long-term storage -70 ℃). 200μl of chloroform was added per 1ml TRIzol stand vigorous shaking after mixing at room temperature was naturally 3-5min phase. 4 ℃ 12,000rpm ...
Embodiment 3
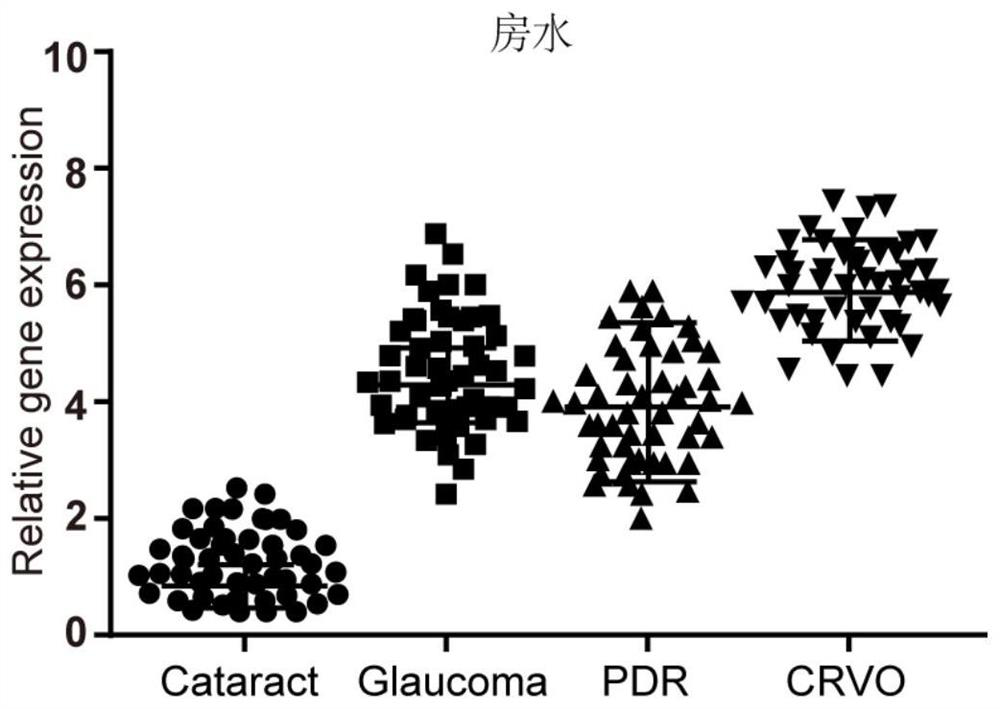
[0075] Example 3 circRNA-Wdr37 feasibility embodiment as prognostic markers
[0076] First step; get blood sample to be inspected
[0077] Serum each disease in each of 200 patients with ischemic retinopathy (primary open-angle glaucoma, central vein occlusion and diabetic retinopathy) collected before and after treatment.
[0078] Step 2: Extract RNA from the blood sample to be inspected
[0079] a) serum samples or frozen serum samples taken 0.25ml transferred into a centrifuge tube, add 1ml Trizol, using a pipette by pipetting up and down repeatedly until the cells were completely lysed.
[0080] b) adding chloroform (+ Trizol Reagent sample liquid volume of the amount of 1 / 5 volume) centrifuge tube cap tightly, shake vigorously 15sec, 5min standing at room temperature;
[0081] c) 4 ℃ centrifugation, 12,000g × 15min, carefully removed from the centrifuge tube, then homogenate is divided into three, namely: the supernatant was colorless, white intermediate layer and a lower lay...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com