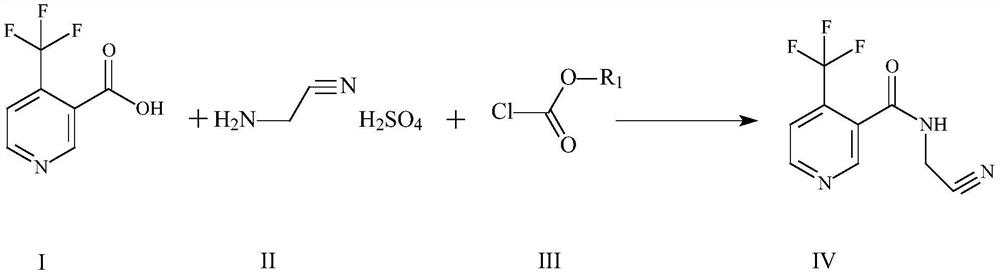
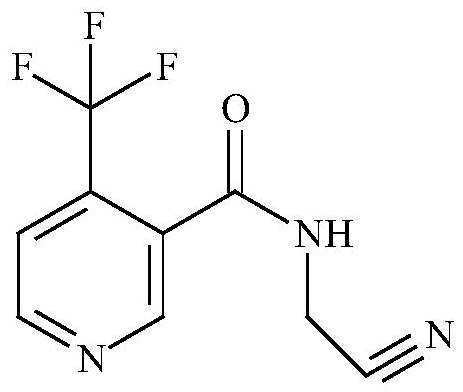
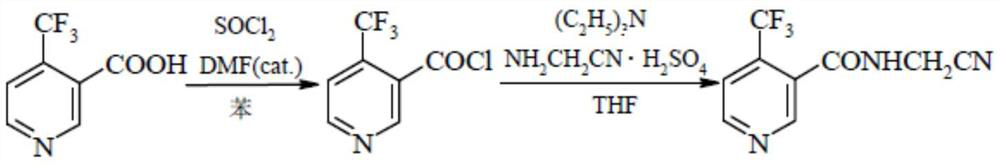Method for preparing flonicamid
A technology of flonicamid and amidation reaction, which is applied in the field of medicinal chemistry, can solve problems such as unsuitable for industrialized production, difficult to realize industrialized, unfavorable for industrialized production, etc., and achieve significant application value, low risk, and simplified operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The specific steps for preparing flonicamid in this example are: add (95.5g, 0.5mol) compound of formula I and (94.5g, 0.5mol) compound of formula III in a 500ml four-necked reaction flask, and add as organic solvent A Dichloromethane 150ml, cool down to 0-10°C in an ice-water bath, and slowly add triethylamine (50.55g, 0.5mol) as an acid-binding agent dropwise at a controlled temperature of 0-10°C, and add (105g, 0.5mol) after the addition is complete Formula II compound, heat up to 30-35 ° C for 4 hours, add 50 g of water dropwise after the reaction is complete, separate layers, adjust the organic layer to PH = 7-8 with liquid caustic, then separate layers, distill the organic layer under reduced pressure, and distill After completion, add 250ml of absolute ethanol as organic solvent B, slowly cool down to 0-5°C, filter and dry with suction to obtain 206.1g of the target compound IV as a dry product, with a molar yield of more than 90% and a purity of more than 98%.
Embodiment 2
[0053]The specific steps for preparing flonicamid in this example are: add (95.5g, 0.5mol) compound of formula I and (54.26g, 0.5mol) compound of formula III in a 500ml four-necked reaction flask, and add as organic solvent A Dichloroethane 150ml, cool down to 0-10°C in an ice-water bath, slowly add triethylamine (50.55g, 0.5mol) dropwise as an acid-binding agent at a controlled temperature of 0-10°C, and drop (105g, 0.5mol) ) Formula II compound, warming up to 30-35°C for 4 hours, adding 50g of water dropwise after the reaction is complete, layering, adjusting the organic layer to PH=7-8 with liquid caustic, then layering, and distilling the organic layer under reduced pressure, After the distillation, add 250ml of absolute ethanol as organic solvent B, slowly cool down to 0-5°C, filter and dry to obtain 207.1g of the target compound IV as a dry product, with a molar yield of over 90.4% and a purity of over 98%.
Embodiment 3
[0055] The specific steps for preparing flonicamid in this example are: add (95.5g, 0.5mol) compound of formula I and (54.26g, 0.5mol) compound of formula III in a 500ml four-necked reaction flask, and add as organic solvent A Toluene 150ml, cool down to 0-10°C in an ice-water bath, and slowly add triethylamine (50.55g, 0.5mol) as an acid-binding agent dropwise at a controlled temperature of 0-10°C, and then add (105g, 0.5mol) of formula II Compound, heat up to 30-35°C and keep warm for 4 hours. After the reaction is complete, 50g of water is added dropwise, and the layers are separated. The organic layer is adjusted to PH=7-8 with liquid caustic soda, and then the layers are separated. The organic layer is distilled under reduced pressure, and the toluene is evaporated. After 100ML, slowly lower the temperature to 0-5°C, filter and dry with suction to obtain 205g of the target compound IV as a dry product, with a molar yield of over 89.5% and a purity of over 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com