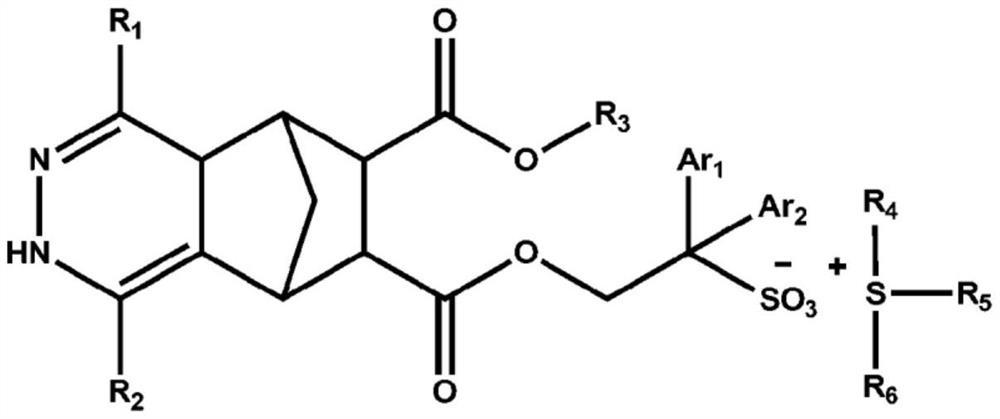
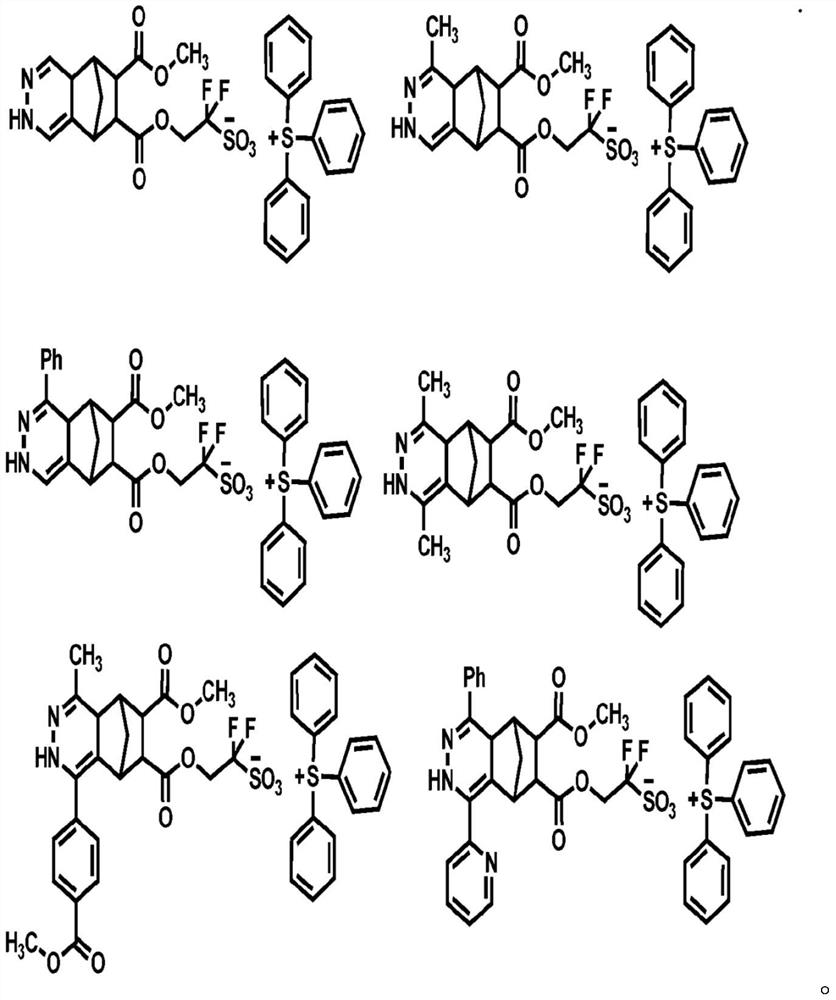
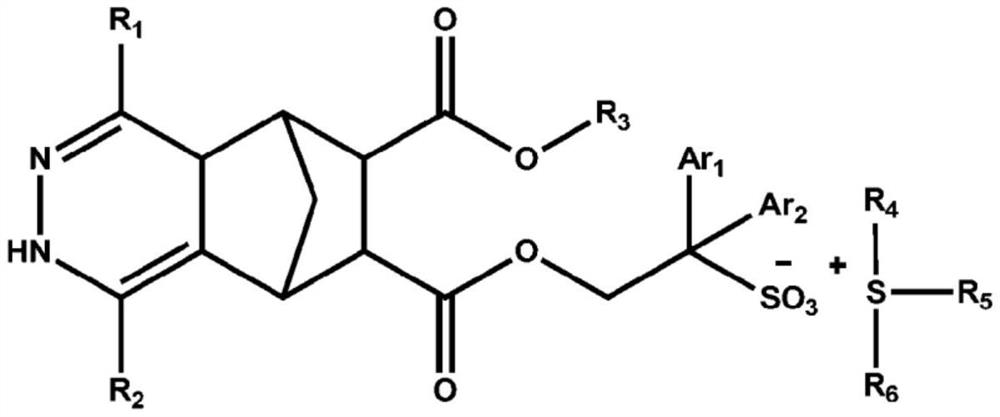
Compound, photoresist composition comprising same, photoresist pattern comprising same, and method for manufacturing photoresist pattern
A technology of photoresist, photoresist layer, applied to compound, photoresist composition containing same, photoresist pattern containing same and for manufacturing photoresist In the field of patterns, it can solve the problems of sensitivity reduction, resolution and line width roughness, etc., and achieve the effect of improving line width roughness, high resolution, and enhancing contrast
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0229] Hereinafter, the specification will be described in detail with reference to the embodiments to specifically describe the specification. However, the embodiments according to the present specification can be modified into various other forms, and the scope of the present specification should not be construed as being limited to the embodiments described below. The embodiments of this specification are provided to more fully describe this specification to those skilled in the art.
preparation example
[0231]
[0232]
[0233] In the synthesis of the compounds, the compounds were synthesized using the substituents described in Table 1 below.
[0234] [Table 1]
[0235] R 1
R 2
Yield(%) Preparation Example 1 H H 95 Preparation example 2 CH 3
H 93 Preparation example 3 Ph H 94 Preparation Example 4 CH 3
CH 3
93 Preparation Example 5 CH 3
4-CO 2 CH 3 P-C 6 h 4
90 Preparation example 6 Ph 2-pyridyl 91
[0236] Preparation of Intermediate 1
[0237]
[0238] Cyclopentadiene (132.2g, 2.0mol), 2-carboxyethyl acrylate (130.1g, 1.0mol) and 4-methoxyphenol (2.4g, 0.02mol) were introduced into the high-pressure reactor, and the resulting Reacted at 180 °C for 18 hours, then vacuum distilled to obtain intermediate 1 (190 g, 97%).
[0239] 1H-NMR (CDCl3): (ppm) δ6.3 (dd, H), 6.2 (dd, H), 3.6 (s, 3H), 3.34 (dd, H), 3.29 (dd, H), 3.2-3.1 (m,2H), 1.5(m,H), 1.4-1.3(br,H)
[...
preparation example 1
[0262]
[0263] 1,2,4,5-Tetrazine (4.5 g, 0.055 mol) and Intermediate 3 (30 g, 0.05 mol) were introduced into dichloromethane (1 L), and the resultant was reacted at room temperature for 12 hours. After the reaction was completed, diethyl ether was introduced thereto to form a precipitate, which was filtered and then dried to obtain A1 (31.2 g, 95%).
[0264] 1H-NMR (DMSO-D6): (ppm) δ = 7.5 (d, H), 7.4-7.1 (m, 15H), 5.7 (s, H), 4.5 (m, 2H), 3.7 (s, 3H) , 3.1-2.8(m, 3H), 2.3-2.2(m, H), 2.1-2.0(m, H), 1.7-1.4(m, 2H)
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com