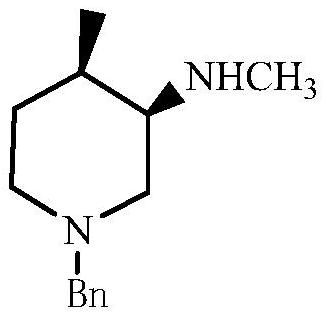
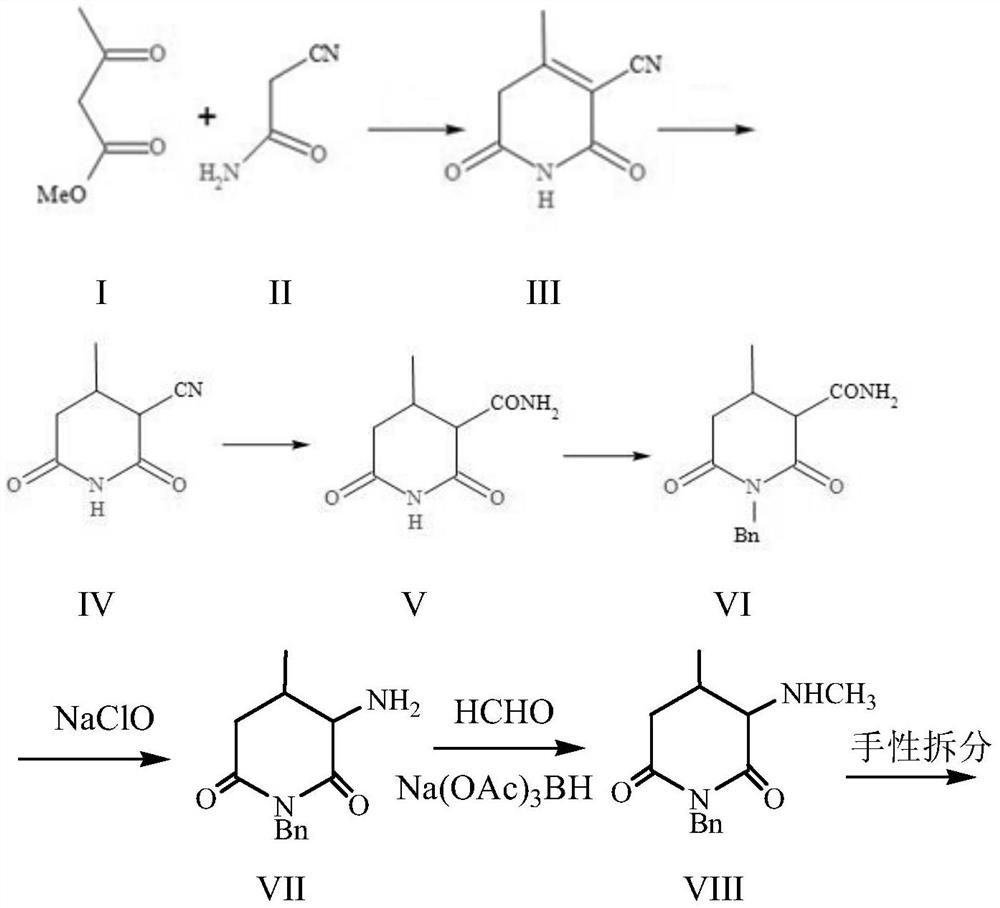
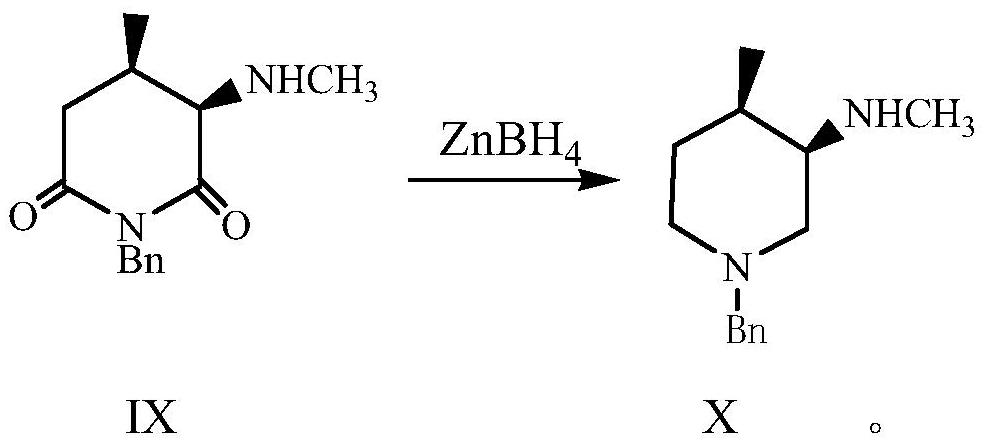
Preparation methods of tofacitinib intermediate amine and dihydrochloride thereof
A technology of tofacitinib and body amine, which is applied in the field of preparation of tofacitinib intermediate amine and its dihydrochloride, can solve the problem of low reaction selectivity, and achieves easy industrialization, easy purification and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Synthesis of 2-cyano-3-methyl-2-ene-glutarimide (compound III): Take 116.0 g (1.0 mol) of methyl acetoacetate and 84.0 g (1.0 mol) of cyanoacetamide, mix , add dropwise methanol solution of sodium methoxide at 20°C under temperature control [methanol solution of sodium methoxide, obtained by dissolving 65.0g (1.2mol) of sodium methoxide in 300mL of methanol], after the addition is complete, gradually raise the temperature to 40-50°C for reaction After 4 hours, the reaction was completed, cooled to 20°C, added dropwise 10% hydrochloric acid aqueous solution to bring the pH value to 3-4, recovered methanol under reduced pressure, cooled to below 10°C to crystallize, filtered, and air-dried to obtain 145.2 g of a yellow solid , dissolved in an aqueous ethanol solution with an ethanol volume concentration of 80% at 70-75 ° C, added activated carbon for decolorization, filtered, cooled and crystallized, and dried under reduced pressure at 50 ° C to obtain 118.5 g of white sol...
Embodiment 2
[0070] Synthesis of 2-cyano-3-methylglutarimide (compound IV): Weigh cyano-3-methyl-2-ene-glutarimide (compound III) 100.0g, use ethanol volume Dissolve 500mL of ethanol aqueous solution with a concentration of 95%, add 6.0g of palladium carbon with a palladium mass content of 5%, put it into a hydrogenation kettle, replace the air completely with nitrogen, and replace it with hydrogen again, press in hydrogen to 0.1Mpa, and control the temperature Hydrogenate at 20-30°C. After 2 hours of reaction, raise the hydrogen pressure to 0.5 MPa and continue the reaction for 1 hour. After the reaction is complete by sampling HPLC, filter to remove palladium carbon, concentrate the filtrate under reduced pressure, add 300 mL of n-heptane to the residue, and stir Crystallize, filter, and dry under reduced pressure at 50°C to obtain 96.8 g of white solid compound IV with a molar yield of 96%.
Embodiment 3
[0072] Synthesis of 3-amido-3-methylglutarimide (compound V): Prepare 300 mL of sulfuric acid with a mass concentration of 85% with concentrated sulfuric acid and purified water, and add 2-cyano group to the sulfuric acid solution in batches under stirring. -3-Methylglutarimide (compound IV) 76.0g (0.5mol), make the amount of the substance of sulfuric acid solute in the sulfuric acid solution be 1.3 times of the amount of substance of compound IV, complete feeding, be warming up to 65~70 Stir at ℃ for 2 hours, the reaction is completed, the reaction solution is cooled to below 40 ℃, the reaction solution is poured into 1000g of crushed ice, the solid is precipitated, filtered, the filter cake is washed with purified water, and blown and dried at 60 ℃ to obtain 76.3g of the compound V, molar yield 89.9%. After the detection purity is greater than 96%, it is directly put into the next step reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com