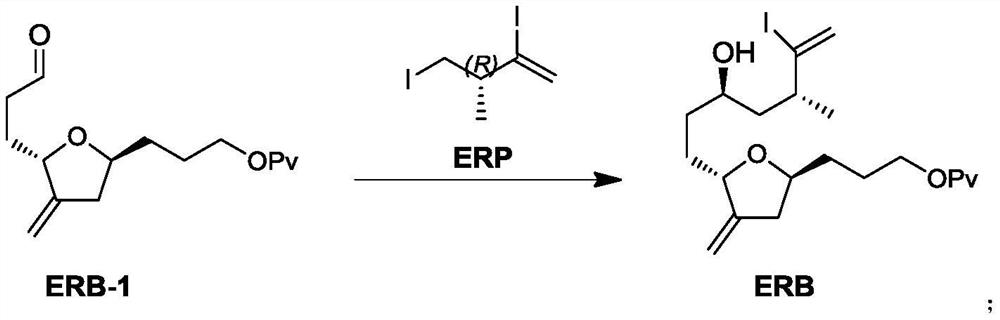
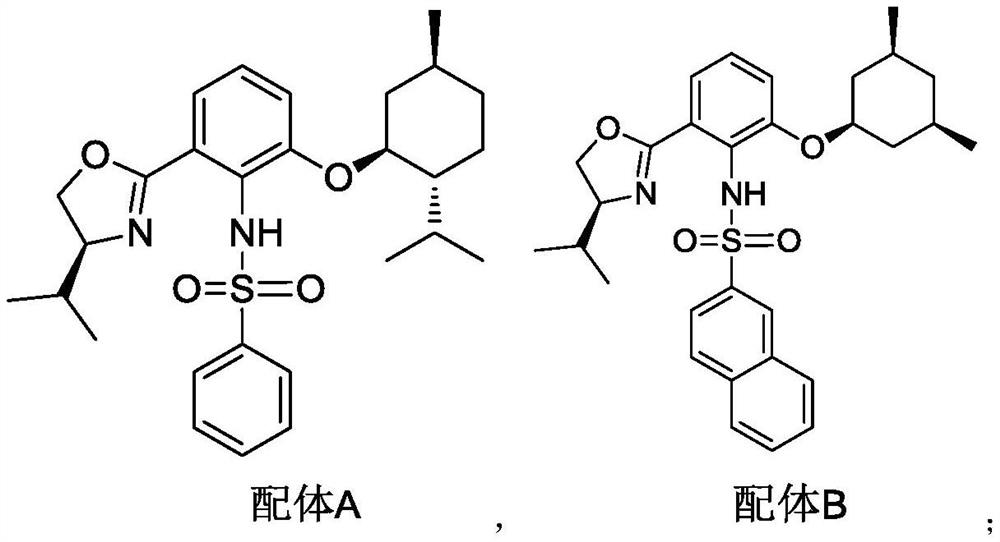
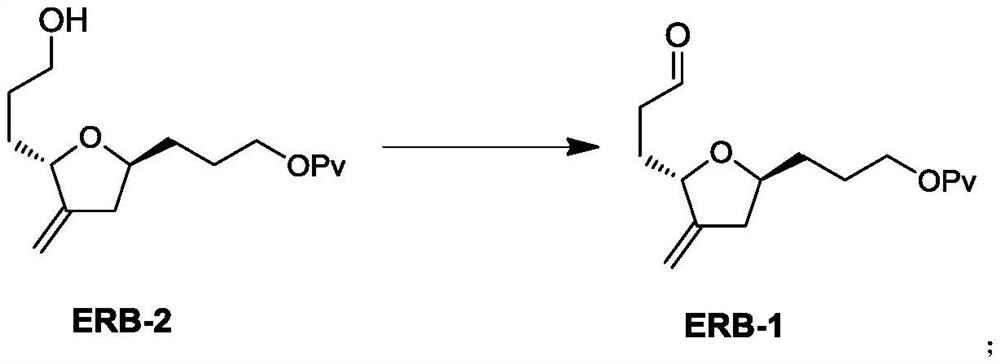
Preparation method of eribulin intermediate
A technology for ligands and compounds, applied in the field of medicine, can solve the problems of high synthesis cost, harsh reaction conditions, and unsuitable for industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1: the preparation of compound ERB-5a
[0071]
[0072] ERB-6 (5 g, 11.4 mmol) was dissolved in 50 mL of dry pyridine, and 4-methoxytriphenylchloromethane (4.58 g, 14.8 mmol) was added with stirring. After the addition, the reaction solution was stirred at room temperature for 17 hours. Add 20 milliliters of ethanol to quench the reaction after TLC detects that the reaction is complete. After the solvent is removed by rotary evaporation, the crude product is dispersed in 40 milliliters of saturated aqueous sodium bicarbonate and 100 milliliters of dichloromethane, left to separate liquids, and the organic phase is dried and concentrated to the column layer Analysis gave 7.62 grams of product ERB-5a, with a yield of 94%.
Embodiment 2
[0073] Embodiment 2: the preparation of compound ERB-4a
[0074]
[0075] ERB-5a (6 g, 8.44 mmol) was dissolved in 50 ml of anhydrous tetrahydrofuran, and tetrabutylammonium fluoride (1M in THF, 11 ml, 11 mmol) was added under stirring at room temperature under nitrogen atmosphere , the reaction was continued for 16 hours. After the reaction was detected by TLC, 50 ml of saturated ammonium chloride aqueous solution was added, followed by extraction with tertiary methyl ether; the organic phases were combined, dried, concentrated, and purified by column chromatography to obtain 3.82 g of ERB-4a with a yield of 96%.
Embodiment 3
[0076] Embodiment 3: the preparation of compound ERB-3a
[0077]
[0078] ERB-4a (3 g, 6.35 mmol) and DMAP (44.4 mmol) were dissolved in 40 ml of dichloromethane, ice water cooling reaction system to 0 ~ 5 ° C, then dropwise added pivaloyl chloride (4.98 g, 41.27 mmol). Return to room temperature after the dropwise addition is complete, stir; add after the completion of the TLC detection reaction, add dichloromethane and sodium bicarbonate aqueous solution, after liquid separation, the organic phase is washed with salt water, after concentration, obtain 3.25 grams of ERB-3a, yield 92%.
[0079] Embodiment 3: the preparation of compound ERB-2
[0080]
[0081] ERB-3a (2.5 g, 4.49 mmol) was dissolved in 20 ml of dichloromethane, cooled to 0 ° C, and 20 ml of 4% methanol solution of p-toluenesulfonic acid was added dropwise thereto under stirring, and TLC detected that the reaction was complete, adding The aqueous solution of sodium bicarbonate was adjusted to pH=7-8, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com