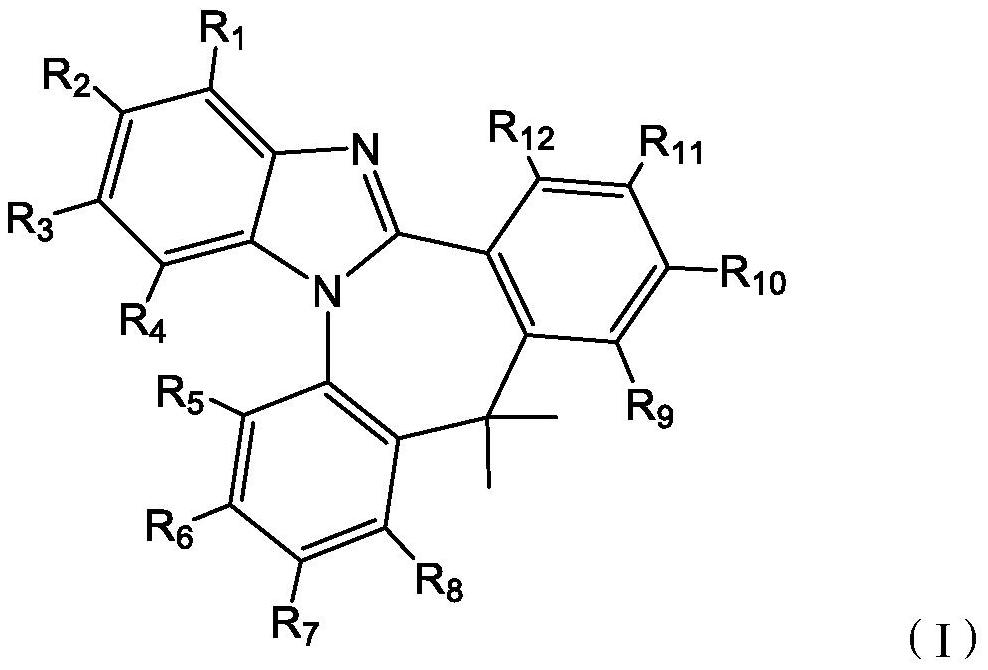
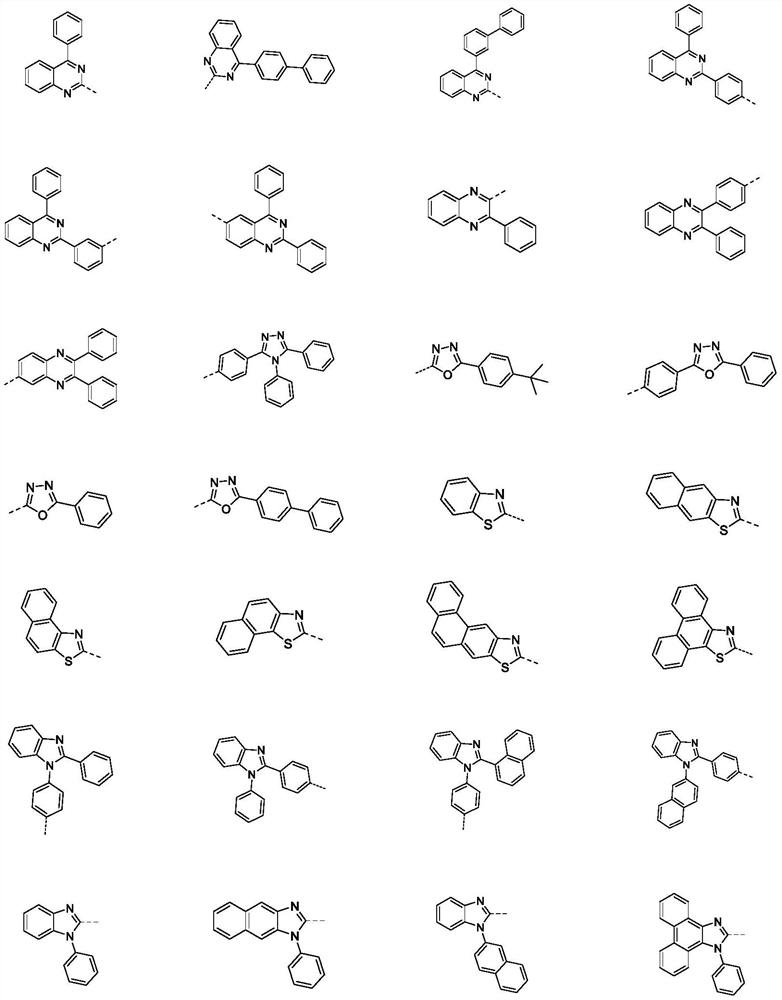
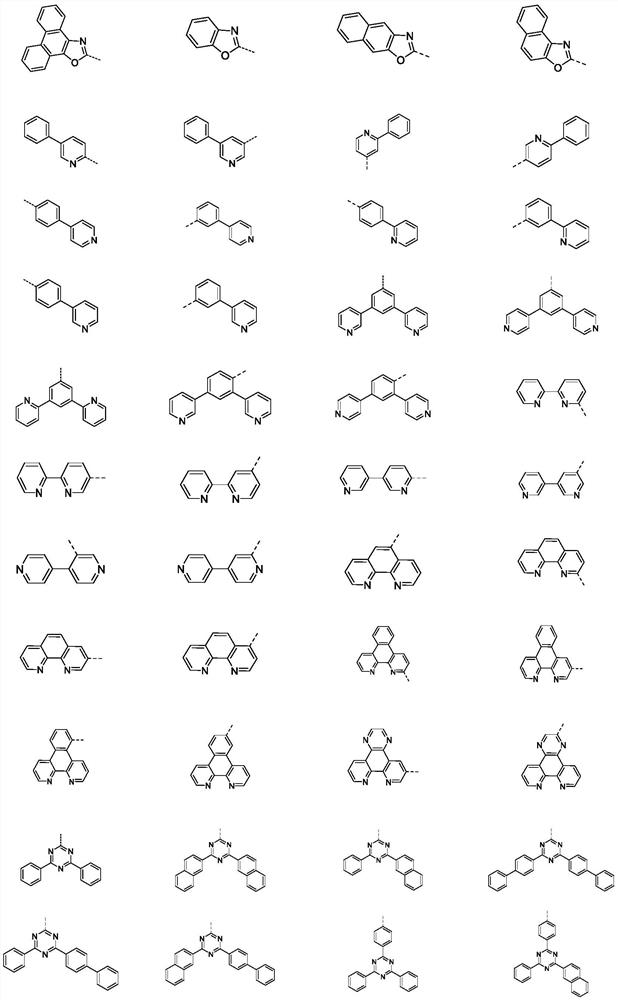
Compound containing polyheterocycles and application
A compound and heterocyclic technology, applied in the field of organic electroluminescence materials, can solve the problems of reduced lifespan, low electron mobility, and high device operating voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] According to the preparation method provided by the present invention, those skilled in the art can use known common means to realize, such as further selecting suitable catalysts, solvents, determining suitable reaction temperature, time, material ratio, etc., the present invention is not particularly limited to this . Unless otherwise specified, the solvents, catalysts, bases and other raw materials used in the preparation process can be synthesized through open commercial channels or methods known in the art.
[0057] Synthetic intermediates M1~M10
[0058] Synthesis of Intermediate M1
[0059]
[0060] The synthetic route is as follows:
[0061]
[0062] The specific operation steps are:
[0063](1) In a 2L three-necked flask equipped with mechanical stirring, add 4-chloro-1-fluoro-2-nitrobenzene (17.5g, 0.1mol), 2-bromo-4-chloroaniline (30.8g, 0.15mol ), stirred, protected by argon, heated up to 180°C, and kept warm for more than 30 hours. During the reac...
Embodiment 1
[0111]
[0112] The synthetic route is as follows:
[0113]
[0114] The synthesis of compound 1-6 comprises the following specific steps:
[0115] Take a 2L three-neck flask, equip it with magnetic stirring, add M1 (41.2g, 0.1mol), (4-phenylquinazolin-2-yl) boric acid (75.0g, 0.3mol), cesium carbonate (117g, 0.36mol) and 800ml of dioxane, start stirring. After nitrogen replacement again, (2.2 g, 11 mmol) tri-tert-butylphosphine and (4.1 g, 4.5 mmol) tris(dibenzylideneacetone) dipalladium were added. After the addition, heat up to raise the temperature, control the temperature at 80-90°C for 4 hours, and cool down after the reaction. Adjust to neutrality, separate the organic phase, extract, dry, perform column chromatography, and spin dry the solvent to obtain 70.1 g of a light yellow solid with a yield of about 76%.
[0116] Product MS (m / e): 922.35; Elemental analysis (C 64 h 42 N 8 ): theoretical value C: 83.27%, H: 4.59%, N: 12.14%; measured value C: 83.03%, H...
Embodiment 2
[0118]
[0119] The synthetic route is as follows:
[0120]
[0121] The synthesis of compound I-16: replace M1 with M2, benzo [d] thiazol-2-yl boronic acid replaces (4-phenylquinazolin-2-yl) boronic acid, select the appropriate material ratio, other raw materials and steps are all Same as Example 1, 49.5 g light yellow solid was obtained, the yield was about 86%.
[0122] Product MS (m / e): 576.14; Elemental analysis (C 36 h 24 N 4 S 2 ): theoretical value C: 74.97%, H: 4.19%, N: 9.71%; measured value C: 74.71%, H: 3.96%, N: 9.48%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com