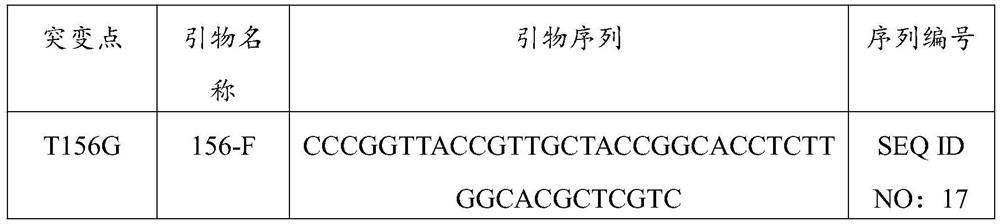
Nicotinamide phosphoribosyltransferase mutant, recombinant expression vector and recombinant bacterium containing mutant, and application
A technology of ribose phosphoric acid and recombinant carrier, applied in the field of biotechnology, can solve the problems of low activity, large amount of organic solvent, difficult separation and purification, etc., and achieve the effects of improving enzyme activity, promoting synthesis and improving enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] The construction of embodiment 1 wild-type Nampt expression vector
[0072] According to the coding sequence of Luteibactersp.UNCMF366Tsu5.1 nicotinamide phosphoribosyltransferase in the GenBank database (GenBank: SFW68293, SEQ ID NO: 36), Suzhou Jinweizhi Biotechnology Co., Ltd. was commissioned to synthesize the gene fragment and named it Nampt gene. In order to facilitate subsequent cloning, two bases of CC were added to the 5' end of the gene fragment to form a NcoI restriction site, and six bases of GGATCC were added to the 3' end to form a BamHI site.
[0073] The synthesized Nampt gene and pET30a vector were double-digested with NcoI and BamHI respectively, and the fragments were recovered with DNA gel kits, ligated with T4 DNA ligase, transformed into Escherichia coli DH5a, and screened with kanamycin-resistant LB plates , to obtain clones.
[0074] Eight clones were picked and the plasmids were extracted with a plasmid extraction kit, sequenced by Suzhou Jinwe...
Embodiment 2
[0075] The expression of embodiment 2 Nampt and the preparation of enzyme
[0076] The expression vector pET30a-Nampt obtained in Example 1 was transformed into Escherichia coli BL21(DE3) to obtain a recombinant strain capable of highly expressing nicotinamide phosphoribosyltransferase.
[0077] The recombinant strain highly expressing nicotinamide phosphoribosyltransferase was fermented and cultured in a shake flask according to a conventional method. When the OD600 of the fermentation broth reached 0.7, it was induced by adding isopropylthiogalactopyranoside IPTG with a final concentration of 1 mM, and the temperature was lowered to 25 Continue culturing at ℃ for 16 hours, centrifuge at 4000 rpm for 20 minutes, collect the cells, wash twice with 50 mM PBS buffer solution with a pH of 7.4, and obtain cells containing nicotinamide phosphoribosyltransferase.
Embodiment 3
[0078] The determination of embodiment 3 nicotinamide phosphoribosyltransferase activity
[0079] The bacterium containing nicotinamide phosphoribosyltransferase collected in Example 2 was weighed 10 g, and cetyltrimethylammonium bromide CTAB with a final concentration of 0.5% was added to permeabilize the cells, and 100 mM pH of 8.0 was added. PBS buffer to a final volume of 50 mL to obtain a crude nicotinamide phosphoribosyltransferase enzyme solution.
[0080] In the 1mL reaction system, add 5-phosphoribose-1-pyrophosphate PRPP to a final concentration of 5mM, add ATP to a final concentration of 1mM, add magnesium ions to a final concentration of 16mM, add 50μL of crude enzyme solution, and add 100mM PBS buffer with a pH of 8.0 solution to a final volume of 1 mL to obtain a reaction solution.
[0081] The reaction solution was reacted at 37° C. for 30 minutes, 250 μL of the reaction solution was taken out, and 250 μL of 100 mM PBS buffer solution with a pH of 8.0 and 500 μ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com