Preparation method of high-purity lenvatinib mesylate crystal form C
A technology of lenvatinib and methanesulfonic acid, applied in the field of medicine and chemical industry, can solve the problems of unsuitability for industrial production, product purity not up to standard, and high impurity content, and achieve the effects of reducing impurity content, convenient operation and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0047] Preparation Example 1: Preparation of Lenvatinib Free Base
[0048] (a) Preparation of 4-(4-amino-3-chlorophenoxy)-7-methoxy-quinoline-6-carboxamide:
[0049]
[0050] At 70°C, under nitrogen protection, 4.35g of 4-amino-3-chlorophenol hydrochloride, 5.38g of potassium hydroxide aqueous solution (48.5w / w%), 4.40g of 4-chloro-7-methoxy A mixture of methyl-quinoline-6-carboxamide and 40 ml of dimethyl sulfoxide was stirred for 20 h. Add acetone (22ml) / water (44ml) mixture at 55°C, cool down to 5-10°C, filter, wash the filter cake with acetone / water, and dry to obtain 5.87g of intermediate with a yield of 92.1%.
[0051] (b) Preparation of lenvatinib free base:
[0052]
[0053] At -20°C, under nitrogen protection, to 2.60g of 4-(4-amino-3-chlorophenoxy)-7-methoxy-quinoline-6-carboxamide, 1.32g of pyridine, 0.14g Add 2.66 g of phenyl chloroformate to a mixture of water and 20 ml of DMF, then keep stirring for 3 h. Then, 1.94 g of cyclopropylamine was further added ...
Embodiment 1
[0060] Example 1: Lenvatinib mesylate crystal form C
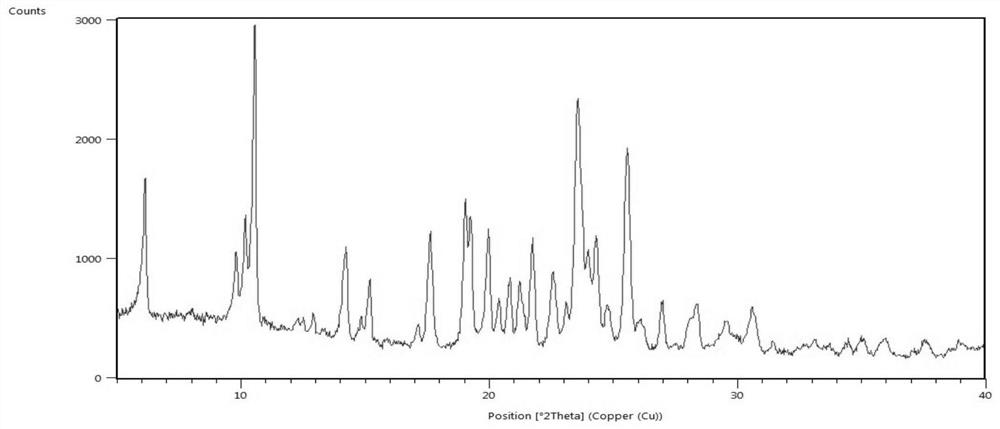
[0061] Add 1.0g of lenvatinib free base to 15ml of ethanol, control the temperature to 15-25°C, add 0.27g of methanesulfonic acid ethanol solution (5ml), keep stirring for 2h, filter with suction, and dry in vacuum at 60°C to obtain 1.17g Lenvatinib mesylate crystal form C, yield 95.5%, HLPC purity 99.9%; After determination, its X-ray powder diffraction pattern is as follows figure 1 As shown, its DSC-TGA spectrum is as image 3 shown.
Embodiment 2
[0062] Embodiment 2: lenvatinib mesylate crystal form C
[0063] Add 1.0 g of lenvatinib free base to 20 ml of ethanol, heat to dissolve, add 0.27 g of methanesulfonic acid at a temperature controlled to 15-25 °C, keep stirring for 3 hours, filter with suction, and dry under vacuum at 60 °C to obtain 1.14 g of methanesulfonic acid Lenvatinib crystal form C, yield 93.1%, HLPC purity 99.8%; After determination, its X-ray powder diffraction pattern and figure 1 Basically the same, its DSC-TGA spectrum and image 3 Basically the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com