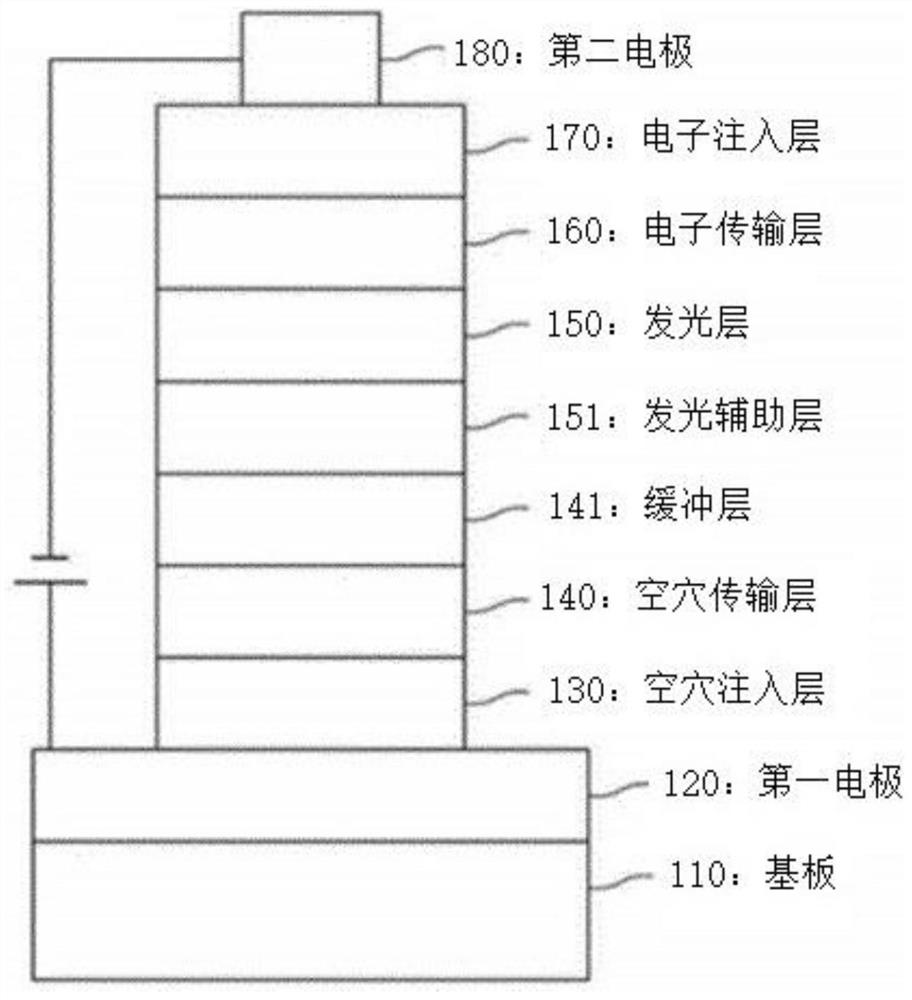
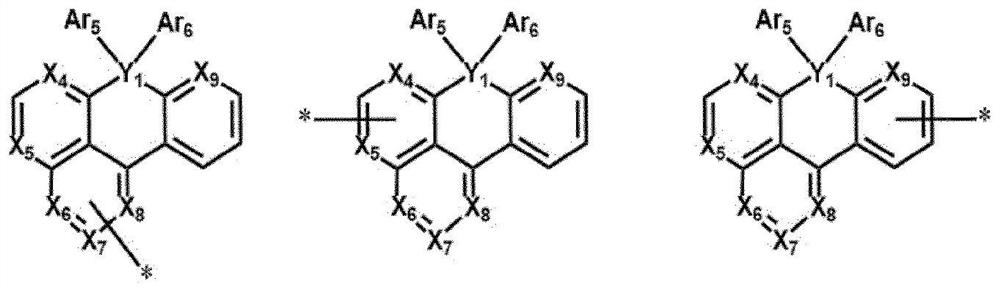
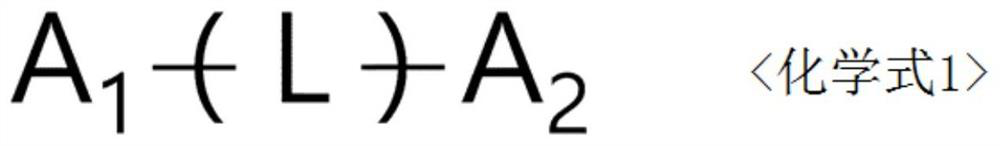Compound, organic light-emitting device and display device
A compound, chemical formula technology, used in organic chemistry, electric solid devices, semiconductor devices, etc., to achieve the effect of excellent hole blocking ability and high electron mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example
[0284] Synthesis Example (Compounds 1-1-1 to 1-1-3)
[0285] After dissolving 2-(benzo[kl]thioxanth-3-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborane (20 g, 55.5 mmol) in THF , add 2-([1,1'-biphenyl]-4-yl)-4-chloro-6-phenyl-1,3,5-triazine (20.9g, 61.1mmol), Pd(PPh 3 ) 4 (2g, 1.7mmol), NaOH (6.8g, 168.9mmol) and water, and then stirred under reflux at 100°C for 3 hours. When the reaction is over, extract with E.A and water, and wash the organic layer with MgSO 4 After drying and concentration, the resulting organic matter was passed through a silica gel column followed by recrystallization, thereby obtaining 20 g of a final product (yield: 67%).
[0286]
[0287] Compounds 1-1-2 to 1-1-3 can be synthesized in the same method as that of Compound 1-1-1 by using Cores 1-2 to 1-3.
[0288] Synthesis Example (Compounds 1-2-1 to 1-2-3)
[0289] After dissolving 2-(benzo[kl]thioxanth-3-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborane (20 g, 55.5 mmol) in THF , added 2-([1,1'-biphenyl]-4-y...
Embodiment
[0552] Example (compound 31-3-9)
[0553] 1-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[4,5]thiochromene[2,3-b]pyridine ( 20g, 55.4mmol) was dissolved in THF, and 1-bromo-4-iodobenzene (17.2g, 60.9mmol), Pd(PPh 3 ) 4 (2g, 1.7mmol), NaOH (6.6g, 166.2mmol) and water, and then stirred under reflux at 100°C for 3 hours. When the reaction is over, extract with E.A and water, and wash the organic layer with MgSO 4 Drying and concentration, then recrystallization after the organic matter that generates is passed through silica gel column, thereby obtain the intermediate product of 15.4g namely 1-(4-bromobenzene) benzo [4,5] thiochromene [2,3-b ] Pyridine.
[0554] After dissolving 1-(4-bromobenzene)benzo[4,5]thiochromene[2,3-b]pyridine (15.4 g, 39.3 mmol) as an intermediate in DMF in a round-bottomed flask, add bis Pinacol borate (11g, 43.2mmol), Pd(dppf)Cl 2 (0.9g, 1.2mmol) and KOAc (16.3g, 117.9mmol), and then stirred under reflux at 130°C for 4 hours. When the reacti...
Embodiment 1~38
[0570] Embodiments 1-38 (Application Examples in Electron Transport Layers of Blue Organic Light-Emitting Devices)
[0571] Corning (corning) 15Ω / cm 2 The ITO glass substrate was immersed in distilled water in which a dispersant was dissolved to be cleaned with ultrasonic waves. The detergent used here was a product purchased from Fischer Co., and the distilled water was distilled water filtered twice using a filter (Filter) purchased from Millipore Co. The ITO was washed for 30 min, followed by two repetitions of ultrasonic washing with distilled water for 10 min. After washing with distilled water, it was followed by ultrasonic washing with isopropanol, acetone, and methanol solvents in sequence, and dried.
[0572] A hole-injection layer with a thickness of 60 nm was formed by vacuum-depositing 2-TNATA on the ITO anode layer, and then vacuum-deposited 4,4-bis[N-(1-naphthyl)-N-benzene on the above-mentioned hole-injection layer Amino]biphenyl (hereinafter referred to as...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com