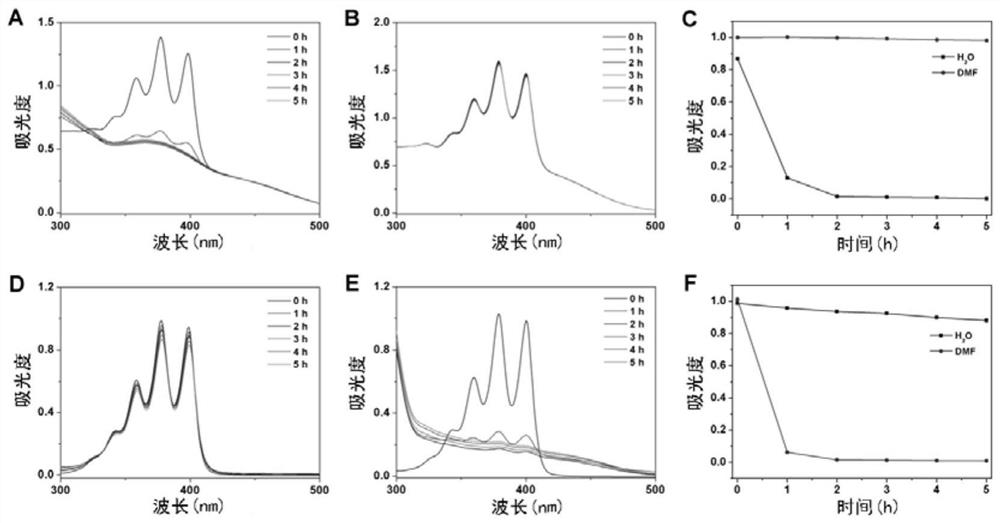
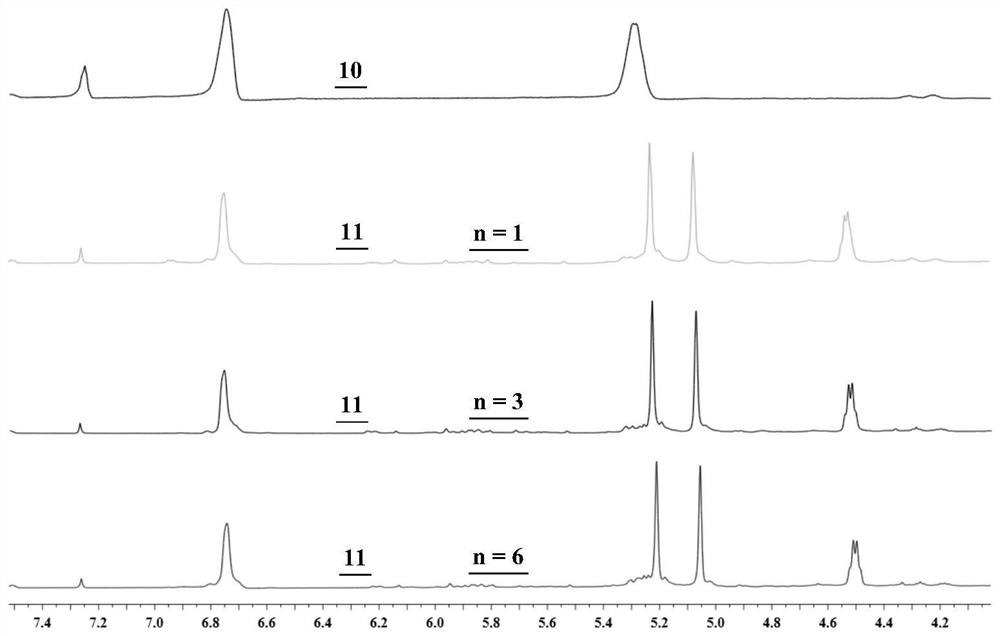
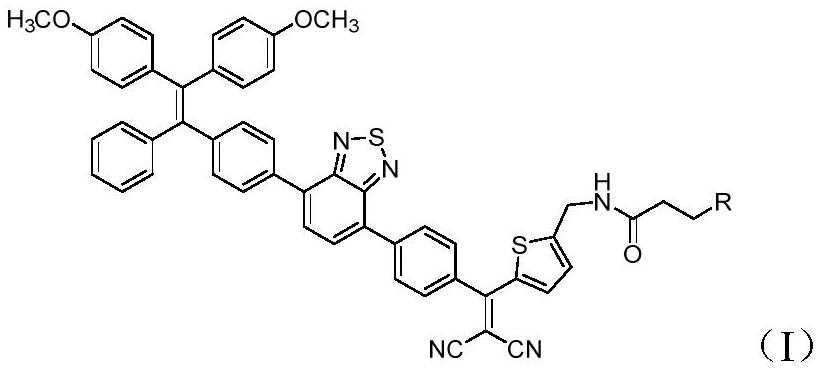
Synthesis and application of photosensitizer
A compound and alkyl technology, which is applied in the synthesis of biologically active molecules or pharmaceutical intermediates, can solve the problems of limiting the effect of photosensitizers and reducing the efficiency of singlet oxygen generation, and achieve the goal of simplifying the post-processing process and improving the utilization rate of photosensitizers Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] (1) Synthesis of compound 3
[0033] Compound 1 (500mg, 0.83mmol), compound 2 (538mg, 1.21mmol), potassium carbonate (2.5M in H 2 (20, 4mL) was added to tetrahydrofuran, tetrakistriphenylphosphine palladium (80mg) was added under nitrogen protection, and reacted at 60°C for 12 hours. The organic layer was extracted, dried, concentrated, and silica gel column chromatography to obtain compound 3 (560mg, 80.5% ). HRMS calcd.for C 51 h 44 N 3 o 5 S 2 [M+H] + 842.2717, found 842.2730; 1 H NMR (500MHz, CDCl 3 )δ8.10(d, J=8.0Hz, 2H), 8.01(d, J=8.0Hz, 2H), 7.85-7.77(m, 4H), 7.60(d, J=3.5Hz, 1H), 7.21( d,J=8.0Hz,2H),7.17-7.08(m,5H),7.07-7.00(m,3H),6.97(d,J=8.5Hz,2H),6.68(d,J=8.5Hz,2H ),6.65(d,J=8.5Hz,2H),5.11(t,J=6.0Hz,1H),4.55(d,J=4.5Hz,2H),3.75(s,3H),3.74(s,3H ),1.49(s,9H); 13 C NMR (126MHz, CDCl 3 )δ187.4, 158.2, 158.1, 155.5, 153.9 (2C), 152.4, 144.7, 144.1, 142.5, 141.2, 140.8, 138.7, 137.4, 136.2, 135.1, 134.6, 133.8, 132.6 (5C), 1391.6, 13 ,129.2...
Embodiment 2
[0043]
[0044] (1) Synthesis of compound V-4
[0045] Compound V-2 (50mg, 0.05mmoL) and HBTU (38mg, 0.10mmoL) were dissolved in 5mL of dichloromethane, added DIEA (36μL, 0.20mmoL) and stirred at room temperature for 10min, then added N-(2-aminoethyl)malein Imide trifluoroacetate (20mg, 0.08mmoL) was reacted at room temperature for 2h. The reaction solution was extracted with dichloromethane, dried, concentrated, and column chromatographed to obtain compound V-4 (46 mg, 82%). HRMS calcd.for C 63 h 51 N 10 o 6 S 2 [M+H] + 1107.3435,found 1107.3456; 1 H NMR (500MHz, CDCl 3 )δ8.14(d, J=7.5Hz, 2H), 7.86(d, J=7.5Hz, 1H), 7.83-7.76(m, 3H), 7.68-7.55(m, 4H), 7.38(s, 1H ),7.20(d,J=7.5Hz,2H),7.17-7.08(m,5H),7.06-7.00(m,3H),6.97(d,J=8.0Hz,2H),6.73-6.62(m, 6H),6.44(s,1H),4.70-4.58(m,4H),3.75(s,3H),3.75(s,3H),3.64(m,2H),3.41(m,2H),3.09(m ,2H),2.79(m,4H).
[0046] (2) Synthesis of compound V-5
[0047] Intermediate V-4 (20mg, 0.018mmoL) was dissolved in DMSO (0.5mL), peptid...
Embodiment 3
[0049]
[0050] Compound V-3 (15mg), compound 5 (100mg, 0.61mmol) was added to acetonitrile (20mL), oxygen was introduced into the reaction solution, light was irradiated for 3 hours (300W, PerfectLight, China), TLC was monitored and filtered after the reaction was completed. The solid compound V-3 was continued to be used in the next photocatalytic oxidation reaction, and the reaction solution was concentrated under reduced pressure to obtain compound 6, which was directly used for NMR determination. HRMS calcd.for C 11 h 17 o 3 [M+H] + 197.1172, found 197.1157; 1 H NMR (300MHz, CDCl 3 )δ8.56(m,1H),6.76(s,1H),5.24(s,1H),5.08(s,1H),4.53(m,1H),2.90-1.83(m,5H),1.77(s ,3H),1.40-1.20(m,3H); 13 C NMR (75MHz, CDCl 3 )δ200.3, 200.1, 151.0, 150.9, 145.2, 145.0, 135.4, 135.3, 112.4, 112.0, 83.2, 83.0, 44.0, 43.8, 37.6, 37.4, 32.6, 32.5, 18.2, 18.0, 15.6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com