Human interleukin-2 variant or derivative thereof
A technology of interleukin and derivatives, applied in the direction of microorganisms, microorganism-based methods, biochemical equipment and methods, etc., can solve the problems of lack of regulatory T cell activation level and reduced IL-2 variants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
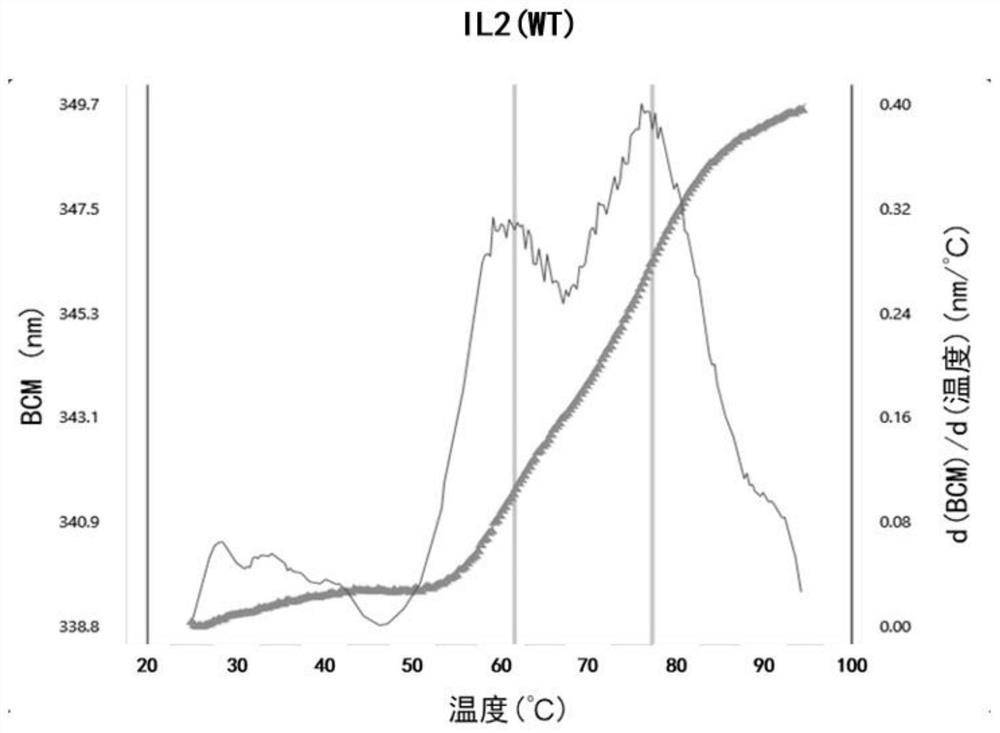
[0159] Embodiment 1: the construction and expression of wild-type IL-2 and variant
[0160] 1. Gene synthesis and recombinant expression vector construction
[0161] The wild-type IL-2 nucleic acid sequence was synthesized by Nanjing GenScript Biotechnology Co., Ltd.
[0162] The synthetic wild-type IL-2 nucleic acid sequence is shown in SEQ ID NO: 1, an Nde I restriction site is added at the 5' end, and a BamH I restriction site is added at the 3' end. The nucleotide sequence is as follows:
[0163] CATATGGCACCGACCAGCAGCAGCACCAAAAAAACCCAGCTGCAACTGGAACATCTGCTGTTAGATCTGCAAATGATTCTGAACGGCATCAACAACTACAAAAATCCGAAACTGACCCGTATGCTGACCTTCAAATTCTACATGCCGAAAAAAGCAACCGAGCTGAAACATCTGCAGTGTCTGGAAGAAGAACTGAAACCGCTGGAAGAGGTTCTGAATCTGGCACAGAGCAAAAACTTTCATCTGCGTCCGCGTGATCTGATTAGCAATATTAACGTTATTGTGCTGGAACTGAAAGGTAGCGAAACCACCTTTATGTGTGAATATGCCGATGAAACCGCAACCATTGTGGAATTTCTGAATCGTTGGATTACCTTTTGTCAGAGCATTATTAGCACCCTGACCTAATGAGGATCC
[0164] SEQ ID NO: 1
[0165] The parts in italics are NdeI and...
Embodiment 2
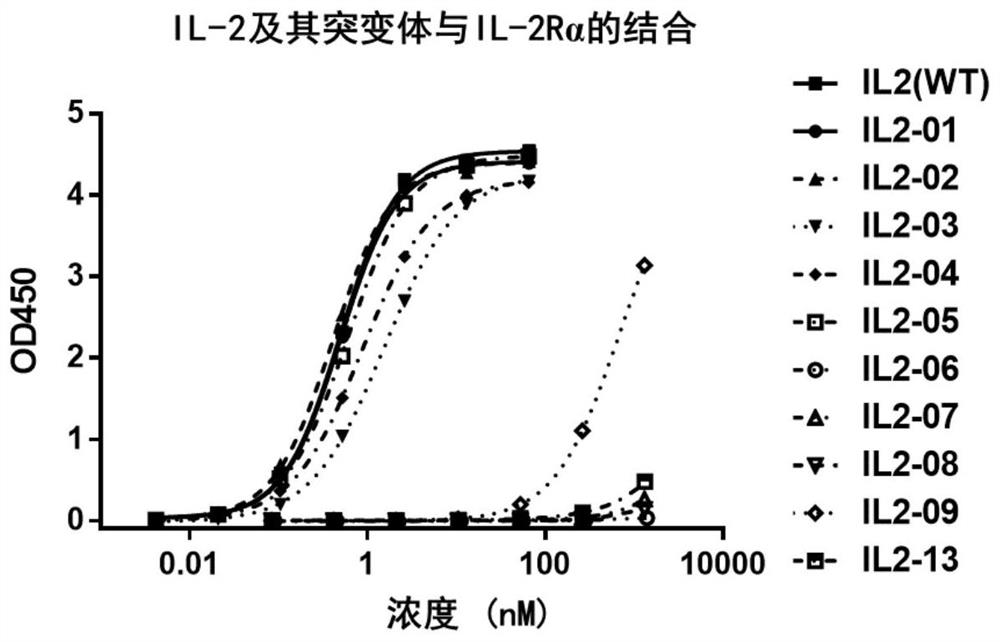
[0289] Embodiment 2: wild-type IL-2 and variants and interleukin 2 receptor alpha (IL-2Rα) binding force determination
[0290] The binding properties of IL-2 and its mutants prepared in Example 1 to IL-2Rα were detected by ELISA experiment. The his-tagged IL-2Rα recombinant protein was coated, and after adding IL-2, the antibody-antigen binding activity was detected by adding HRP-coupled anti-IL-2 polyclonal antibody and HRP substrate TMB.
[0291] A 96-well ELISA plate was coated with 2 μg / mL his-tagged IL-2Rα recombinant protein (SinoBiological, Cat#10165-H08H) and incubated overnight at 4°C. Wash three times with washing solution, 250 μl per well. Shake for 10 seconds per wash to ensure thorough cleaning. Add 200 μl / well blocking solution and incubate at room temperature for 2 hours. Wash three times with washing solution, 250 μl per well. Shake for 10 seconds per wash to ensure thorough cleaning. Add 100 μl of IL-2 and its mutants diluted with diluent to each well. ...
Embodiment 3
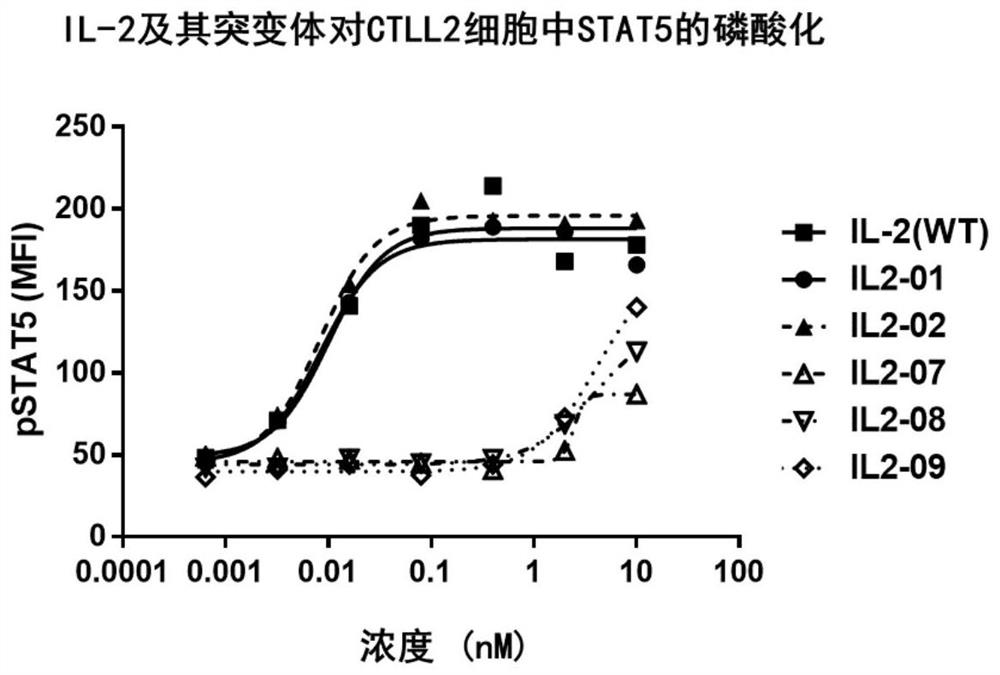
[0297] Embodiment 3: wild-type IL-2 and variants and IL-2 receptor beta / gamma (IL-2Rβ / γ) binding assay Biacore experiment is used to detect IL-2 and mutants thereof and IL-2 in embodiment 1 IL-2Rβ / γ binding.
[0298] First, IL-2Rβ and IL-2Rγ subunits (SEQ ID NO: 41 and 42) were cloned and fused to Fc hole and Fc knob, respectively, for the preparation of tool molecule IL-2Rβ / γ-Fc heterodimer . IL-2Rβ-Fc-hole and IL-2Rγ-Fc-knob were simultaneously transfected into HEK293 cells. The heterodimer was purified with Protein A and molecular sieve Superdex 200 successively.
[0299] MDMRVPAQLLGLLLLWFPGARCAVNGTSQFTCFYNSRANISCVWSQDGALQDTSCQVHAWPDRRRWNQTCELLPVSQASWACNLILGAPDSQKLTTVDIVTLRVLCREGVRWRVMAIQDFKPFENLRLMAPISLQVVHVETHRCNISWEISQASHYFERHLEFEARTLSPGHTWEEAPLLTLKQKQEWICLETLTPDTQYEFQVRVKPLQGEFTTWSPWSQPLAFRTKPAALGKDTGAQDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVCTLPPSRDELTKNQVSLSCAVKGFYPSDIAVEWESNGQPE...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com