Preparation method of polysubstituted pyrroline compound
A technology for dihydropyrroles and compounds is applied in the field of preparation of polysubstituted dihydropyrroles, which can solve complex problems and achieve the effect of mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
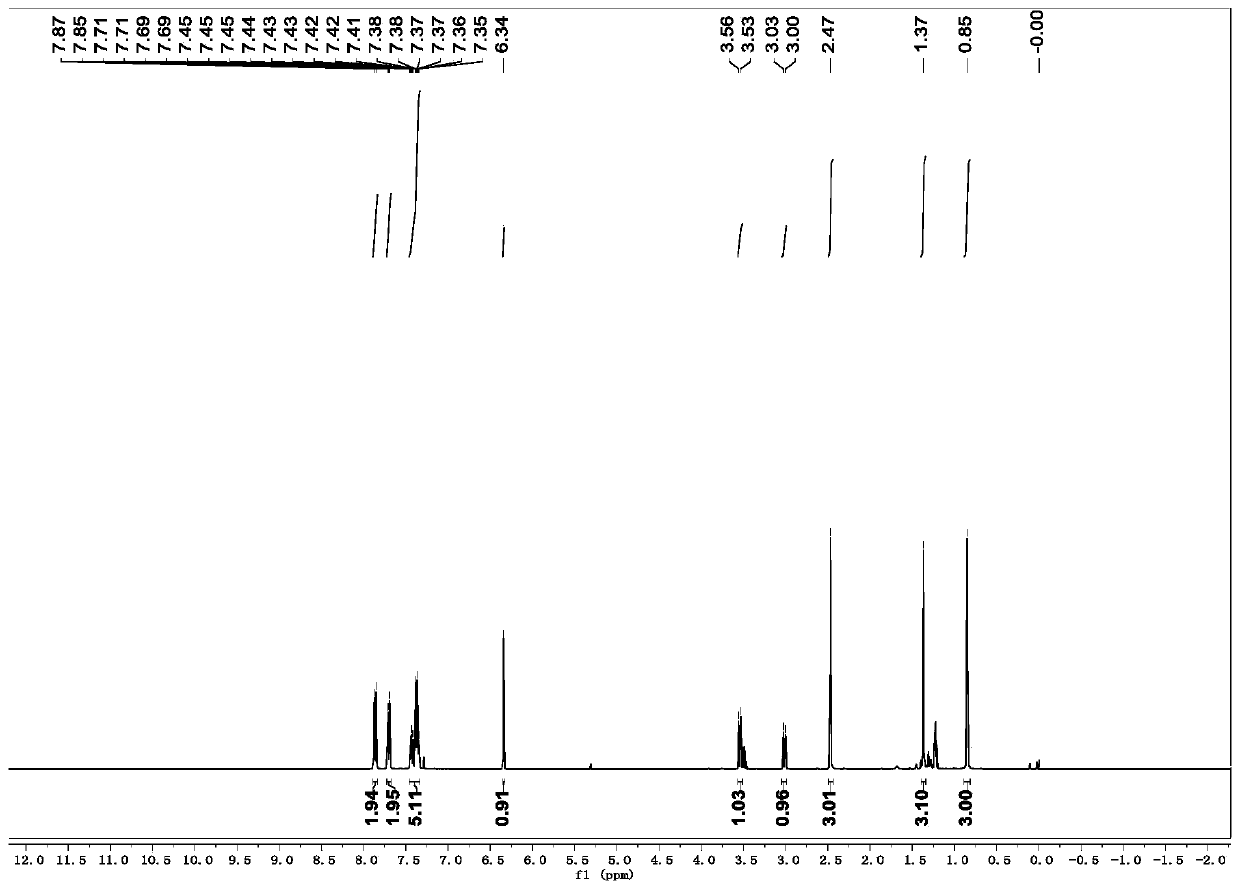
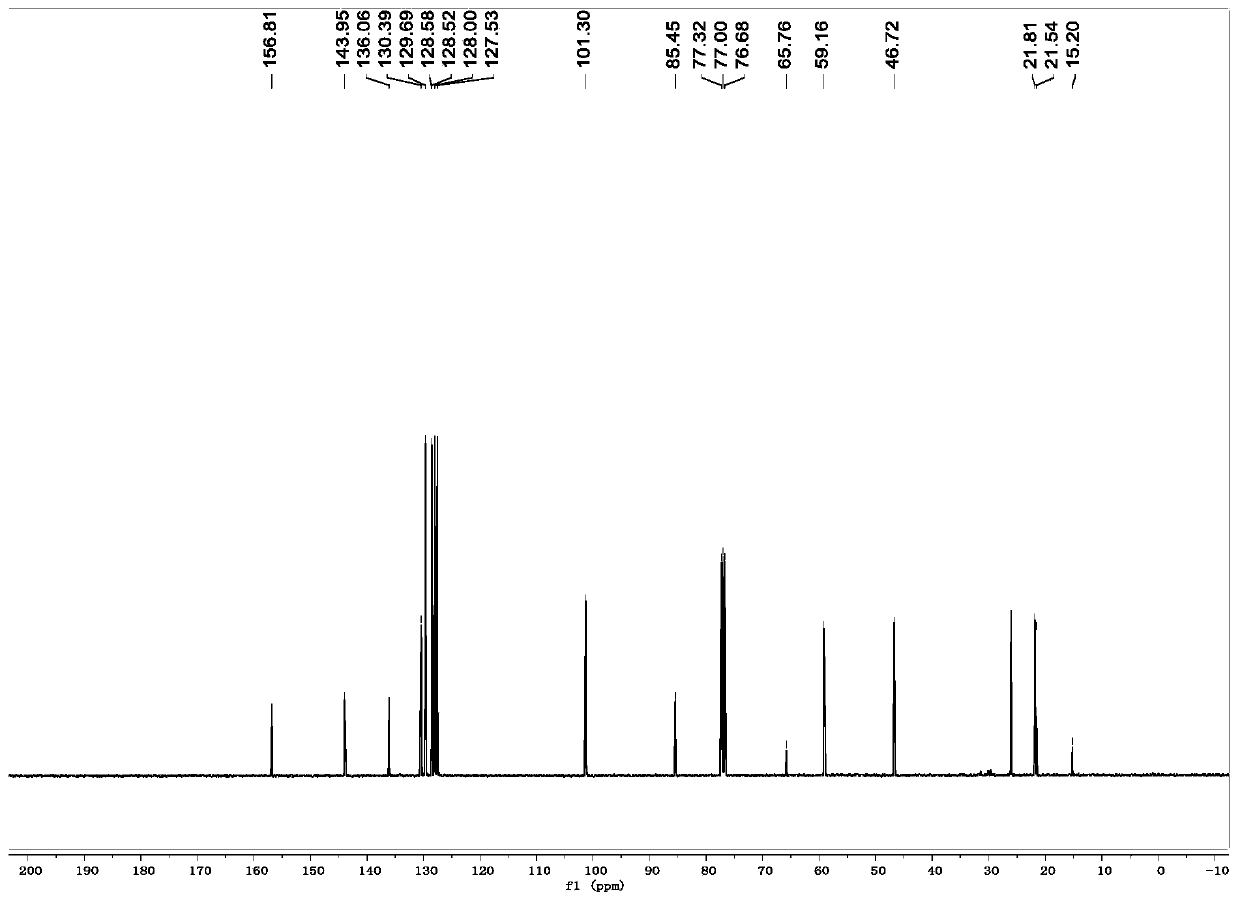
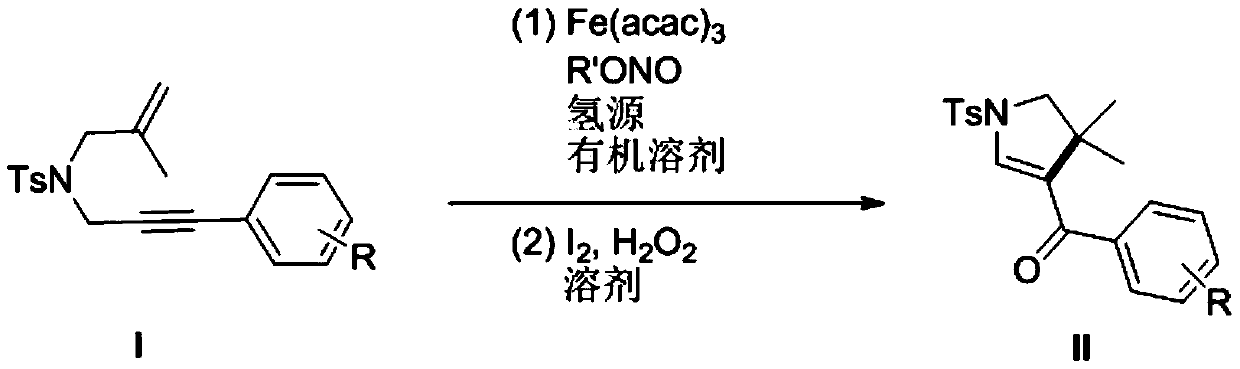
[0035] R is the preparation method of 1-p-toluenesulfonyl-3-benzoyl-4,4-dimethyl-dihydropyrrole with hydrogen:
[0036] (1) In a 10mL dry reaction tube, add raw materials N-2-methylallyl-N-3-phenylpropargyl p-toluenesulfonamide (0.170g, 0.5mmol), Fe (acac) 3 (0.1mmol), tert-butyl nitrite (0.103g, 1mmol), polymethylsiloxane (0.333g, 1.5mmol) and 10mL tetrahydrofuran, and the mixed system was reacted at 60°C for 24 hours. As detected by TLC, the raw material disappeared, and the reaction was completed, and quenched by adding water. The aqueous phase was extracted twice with ethyl acetate (20 mL×2), the combined organic phases were washed once with saturated brine, and the solvent was removed by spinning under reduced pressure to obtain a crude product.
[0037] (2) Next, the crude product was dissolved in 10 mL of acetonitrile-water (9:1 by volume) solution, and a catalytic amount of iodine (12 mg) and hydrogen peroxide (1 mmol) were added, and reacted at room temperature for ...
Embodiment 2
[0040] Wherein R is the preparation method of 1-p-toluenesulfonyl-3-(4-methoxybenzoyl)-4,4-dimethyl-dihydropyrrole of 4-methoxyl group:
[0041] (1) In a 10mL dry reaction tube, add the raw material N-2-methylallyl-N-3-(4-methoxyphenyl)propargyl p-toluenesulfonamide (0.185 g, 0.5mmol), Fe(acac) 3 (0.1mmol), tert-butyl nitrite (0.103g, 1mmol), polymethylsiloxane (0.333g, 1.5mmol) and 10mL tetrahydrofuran, and the mixed system was reacted at 60°C for 24 hours. As detected by TLC, the raw material disappeared, and the reaction was completed, and quenched by adding water. The aqueous phase was extracted twice with ethyl acetate (20 mL×2), the combined organic phases were washed once with saturated brine, and the solvent was removed by spinning under reduced pressure to obtain a crude product.
[0042] (2) Next, the crude product was dissolved in 10 mL of acetonitrile-water (9:1 by volume) solution, and a catalytic amount of iodine (12 mg) and hydrogen peroxide (1 mmol) were adde...
Embodiment 3
[0046] The preparation method of 1-p-toluenesulfonyl-3-(4-fluorobenzoyl)-4,4-dimethyl-dihydropyrrole in which R is 4-fluoro:
[0047] (1) In a 10mL dry reaction tube, add the raw material N-2-methylallyl-N-3-(4-fluorophenyl)-propargyl p-toluenesulfonamide (0.179g , 0.5mmol), Fe(acac) 3 (0.1mmol), tert-butyl nitrite (0.103g, 1mmol), polymethylsiloxane (0.333g, 1.5mmol) and 10mL tetrahydrofuran, and the mixed system was reacted at 60°C for 24 hours. As detected by TLC, the raw material disappeared, and the reaction was completed, and quenched by adding water. The aqueous phase was extracted twice with ethyl acetate (20 mL×2), the combined organic phases were washed once with saturated brine, and the solvent was removed by spinning under reduced pressure to obtain a crude product.
[0048] (2) Next, the crude product was dissolved in 10 mL of acetonitrile-water (9:1 by volume) solution, and a catalytic amount of iodine (12 mg) and hydrogen peroxide (1 mmol) were added, and reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com