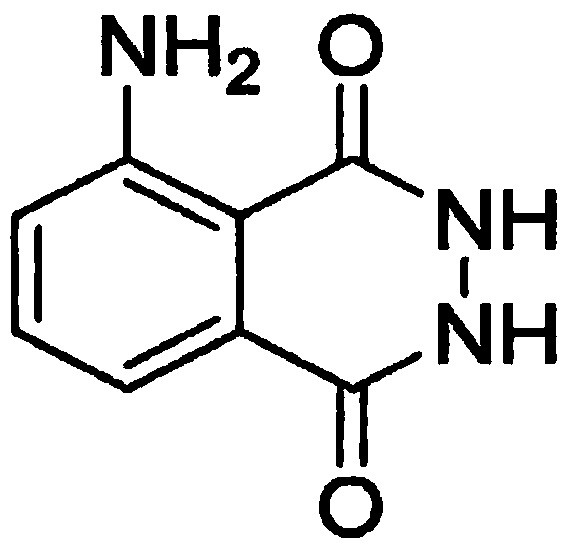
Method for solubilizing 5-amino-2,3-dihydro-1,4-phthalazinedione
A technology of dihydrogen and amino, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of reducing efficacy, and achieve the effect of cheap production costs and cheap materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0234] Example 1: Solubilization of 5-amino-2,3-dihydro-1,4-phthalazinedione - Embodiment 1
[0235] The following indications refer to the weight percent of the mixture. About 100 ml of solubilized material was produced. 5-Amino-2,3-dihydro-1,4-phthalazinedione was provided, and then the solubilizers were mixed one by one with stirring at room temperature (20±5° C.) and atmospheric pressure for 5 minutes.
[0236]
[0237]
[0238] The composition was then carefully heated in temperature increments of about 1°C / minute under continuous stirring. After about 20 minutes (about 40°C), the composition started to turn into a clear solution. This solubilization process lasted over about 16 minutes. Thus, at about 56° C., the solubilized product according to the invention is obtained after about 36 minutes. Heating and stirring were then discontinued, and the resulting solubilized mass was allowed to cool to room temperature. The solubilized material remained clear and st...
Embodiment 2
[0239] Example 2: Solubilization of 5-amino-2,3-dihydro-1,4-phthalazinedione - Embodiment 2
[0240] The following indications refer to the weight percent of the mixture. About 100 ml of solubilized material was produced. 5-Amino-2,3-dihydro-1,4-phthalazinedione was provided, and then the solubilizers were mixed one by one with stirring at room temperature (20±5° C.) and atmospheric pressure for 5 minutes.
[0241]
[0242] The composition was then carefully heated in temperature increments of about 1.5°C / minute with continuous stirring. After about 23 minutes (about 55°C), the composition started to turn into a clear solution. This solubilization process lasted for about 10 minutes or more. Thus, the solubilisate according to the invention is obtained after about 33 minutes at about 70° C. Heating and stirring were then discontinued, and the resulting solubilized mass was allowed to cool to room temperature. The solubilized material remained clear and stable for a m...
Embodiment 3
[0243] Example 3: Solubilization of 5-amino-2,3-dihydro-1,4-phthalazinedione sodium salt
[0244] The following indications refer to the weight percent of the mixture. About 100 ml of solubilized material was produced. Provide 5-amino-2,3-dihydro-1,4-phthalazinedione sodium salt (in the form of the type I polymorph as described in WO 2011 / 107295 A1), then at room temperature (20±5 °C) and atmospheric pressure for 5 minutes to mix the solubilizers one by one.
[0245]
[0246] The composition was then carefully heated in temperature increments of about 1°C / minute under continuous stirring. After about 32 minutes (about 52°C), the composition started to turn into a clear solution. This solubilization process lasted for about 8 minutes or more. Thus, the solubilisate according to the invention is obtained after about 40 minutes at about 60° C. Heating and stirring were then discontinued, and the resulting solubilized mass was allowed to cool to room temperature. The so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com