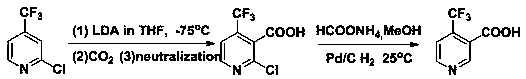
Preparation method of 4-trifluoromethyl nicotinic acid
A technology of trifluoromethylnicotinic acid and synthetic methods, applied in the direction of organic chemistry, etc., can solve the problems of unsuitability for industrial production, long route, low yield, etc., and achieve the effects of low equipment requirements, simple reaction operation, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) N Preparation of -(2-methoxycarbonylvinyl)-4,4,4-trifluoro-3-one-1-butenylamine:
[0039]Methyl acrylate (8.00g, 92.93mmol) was dissolved with 50mL of toluene and 50mL of water, palladium chloride (494.35mg, 2.79mmol) and copper chloride (1.50g, 11.15mmol) were added, and oxygen was introduced into the system, and After the completion of the reaction was monitored by gas chromatography, the system was filtered, and the filtrate was slowly added dropwise to 4-amino-1,1,1-trifluoro-3-buten-2-one (10.00g, 71.9 mmol), and the system was heated and refluxed to react After 4.5 hours, when TLC monitors that the reaction is no longer in progress, adjust the pH of the system to 1.2 with 1mol / L HCl after the reaction is completed, and a large amount of solids are precipitated in the system, filtered by suction, and dried (moisture content to 0.5%) to obtain a light yellow solid product N -(2-methoxycarbonylvinyl)-4,4,4-trifluoro-3-keto-1-butenamine (12.84 g, yield: 80%).
...
Embodiment 2
[0043] On the basis of Example 1, methyl 3-oxopropionate is prepared with methyl acrylate, and then methyl 3-oxopropionate is prepared N -(2-methoxycarbonylvinyl)-4,4,4-trifluoro-3-ketone-1-butenamine, the reaction conditions are: dissolving methyl acrylate with toluene and water, adding catalyst and oxidizing agent, After the reaction is finished, remove the solid by filtration, slowly add the above filtrate dropwise to 4-amino-1,1,1-trifluoro-3-buten-2-ketone, after adding the raw materials, the system is heated and refluxed for 4.5 hours, and the reaction After the end, adjust the pH of the mixed solution to 1.5, and a light yellow solid precipitates out, which is the product N -(2-Methoxycarbonylethenyl)-4,4,4-trifluoro-3-one-1-butenamine.
Embodiment 3
[0045] On the basis of embodiment 1 or 2, N -(2-Methoxycarbonylvinyl)-4,4,4-trifluoro-3-ketone-1-butenamine is hydrolyzed by one-pot ring closure under the action of alkali to obtain 4-trifluoromethylnicotinic acid, The reaction conditions are: sodium methoxide, N -(2-Methoxycarbonylvinyl)-4,4,4-trifluoro-3-keto-1-butenamine and anhydrous methanol were added to the reaction flask in turn, and stirred at room temperature until sodium methoxide and N After -(2-methoxycarbonylvinyl)-4,4,4-trifluoro-3-keto-1-butenamine is completely dissolved, the system is heated and refluxed for 6 hours, cooled to room temperature, and sodium hydroxide aqueous solution is added, Heating and hydrolysis, after the reaction is finished, adjust the pH of the solution to 3, a light yellow solid precipitates out, filter, and dry the filter cake to obtain the product which is 4-trifluoromethylnicotinic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com