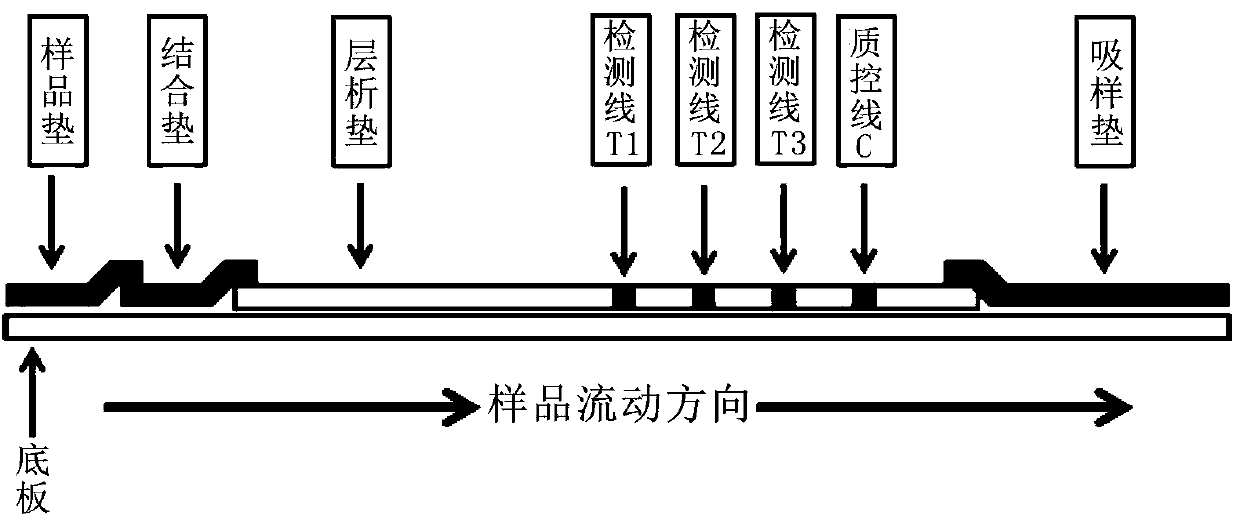
Test strip for esophageal precancerous lesion malignant progress risk detection or esophageal cancer early screening
A risk detection, esophageal cancer technology, applied in the field of medicine and biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Embodiment 1: the preparation of reagent strip
[0080] 1. Experimental materials
[0081] PSMC4 monoclonal antibody (purchased from Santa Cruz Biotechnology Company, product number: sc-166003), ALDOC monoclonal antibody (purchased from Abcam Company, product number: ab87122) and TXLNA monoclonal antibody (Santa Cruz Biotechnology company, product number: sc-80994); PSMC4 polyclonal antibody (Leading Biology Company, catalog number APR07165G), ALDOC polyclonal antibody (Leading Biology Company, catalog number APG01815G), TXLNA polyclonal antibody (LSBio Company, catalog number LS-C314712) and rabbit anti-mouse IgG (Abcam Company, catalog number ab6728).
[0082] PVC bottom plate, nitrocellulose membrane, glass fiber membrane, absorbent paper etc. are all existing commercially available products.
[0083] 2. Preparation of binding pads
[0084] (1) Activation of quantum dots: N-hydroxysulfosuccinimide is used to activate the carboxyl groups on the surface of CdSe / ZnS ...
Embodiment 2
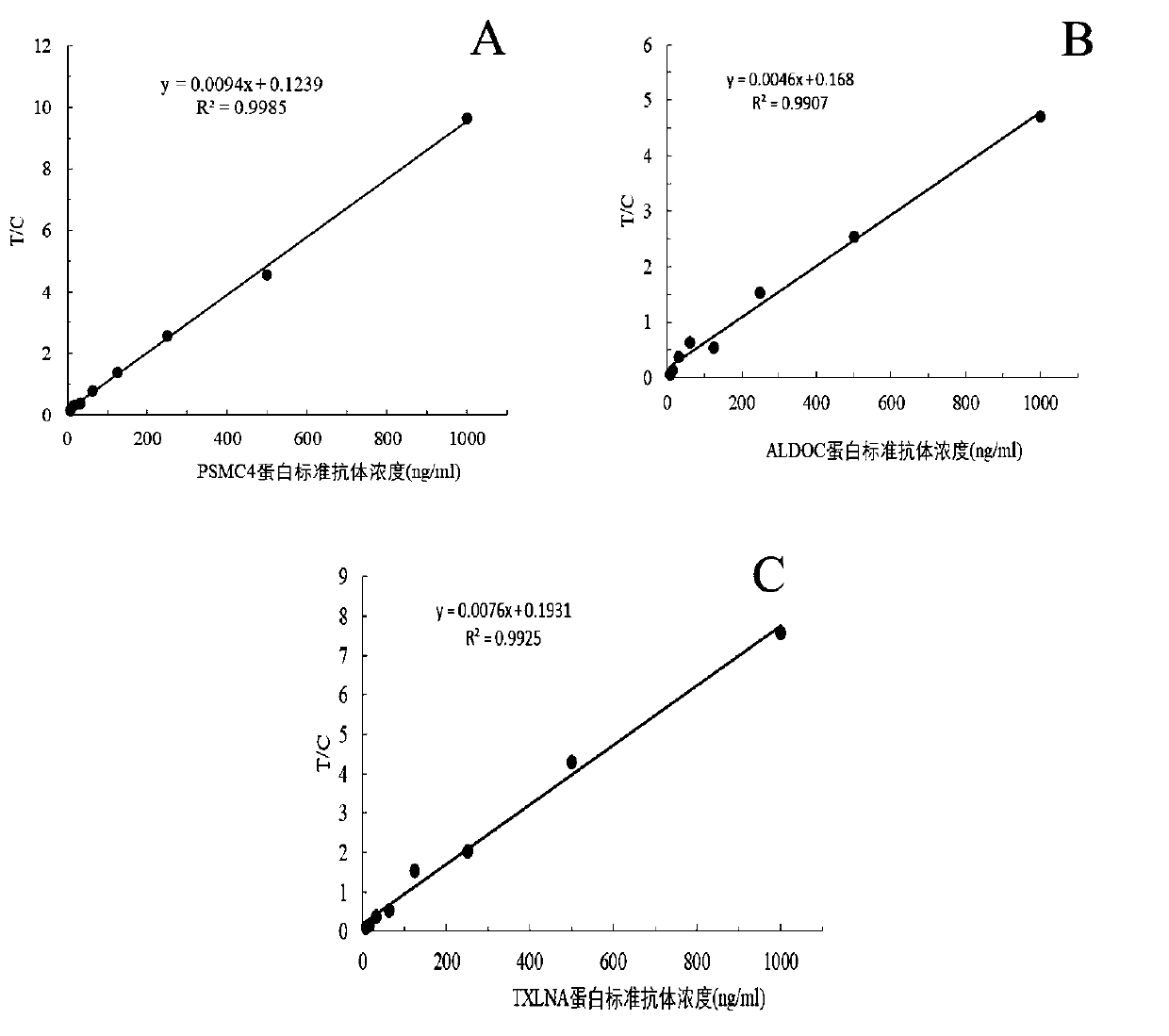
[0102] Example 2: Value analysis of test strips for early screening of esophageal cancer
[0103] The test strips prepared in Example 1 of the present invention were used to detect the serum of patients with esophageal cancer and precancerous lesions to verify the expression levels of PSMC4 gene, ALDOC gene and TXLNA gene in the serum of patients with esophageal cancer and precancerous lesions.
[0104] 1. Sample source
[0105] Serum samples from 400 cases were collected from the Key Open Laboratory of Esophageal Squamous Cell Carcinoma in Henan Province, the First Affiliated Hospital of Zhengzhou University. The 400 serum samples included 200 serum samples from patients with early esophageal cancer (esophageal cancer group) and 200 serum samples from patients with precancerous lesions of the esophagus (precancerous lesion group). The sera of 200 patients with esophageal cancer (esophageal cancer group) were all from newly diagnosed patients who were diagnosed by endoscopy a...
Embodiment 3
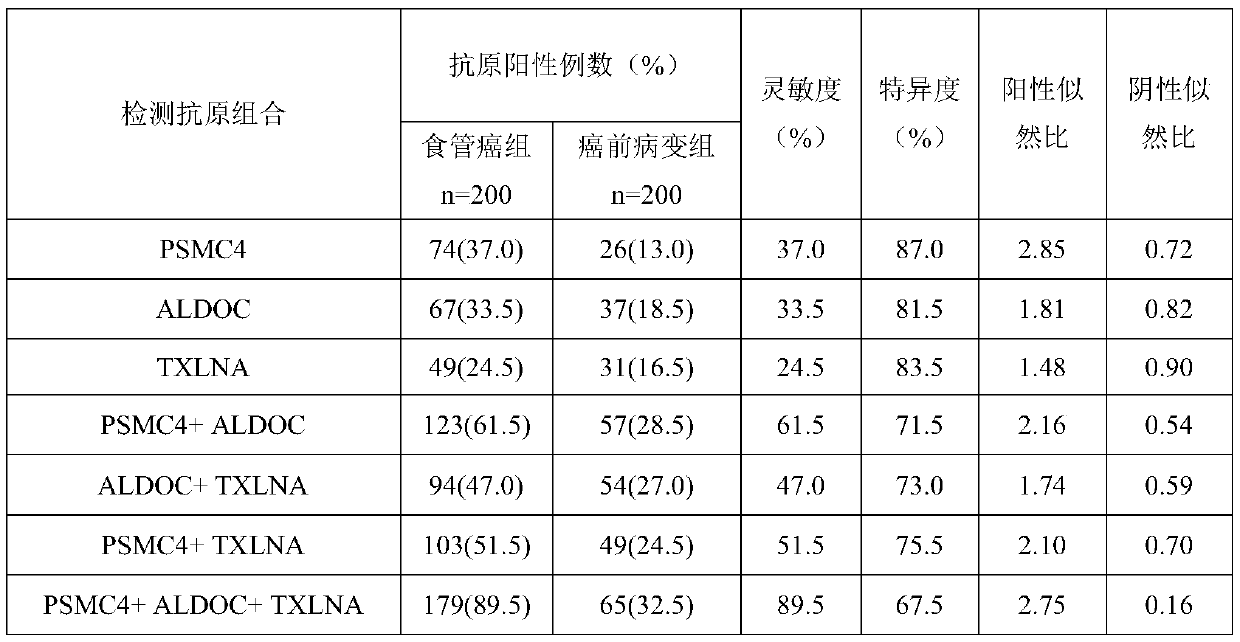
[0116] Example 3: Analysis of the value of test strips for risk detection of malignant transformation of esophageal precancerous lesions
[0117] In order to further study the value of the test strip in the risk detection of malignant transformation of precancerous lesions of esophageal cancer, endoscopic diagnostic tests were selected in high-incidence areas of esophageal cancer (Linzhou City, Anyang City, Huixian City, Henan Province, and Changzhi City, Shanxi Province). For 10,000 patients with precancerous lesions of the esophagus, aged 40-70 years, the peripheral blood of 10,000 patients with precancerous lesions was collected, centrifuged to get serum, and then the test strip prepared in Example 1 of the present invention was used to test 10,000 patients with precancerous lesions The serum is tested, and the high-risk group of canceration (the test strip test result is positive, that is, the high-risk group of canceration) and the non-high-risk group of canceration (the t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com