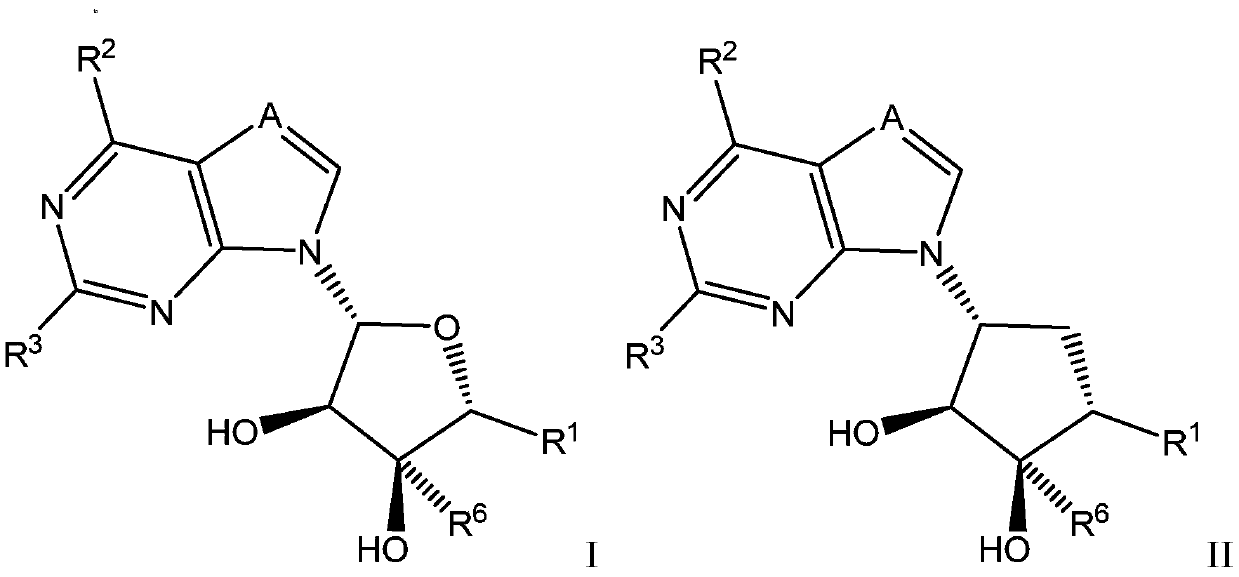
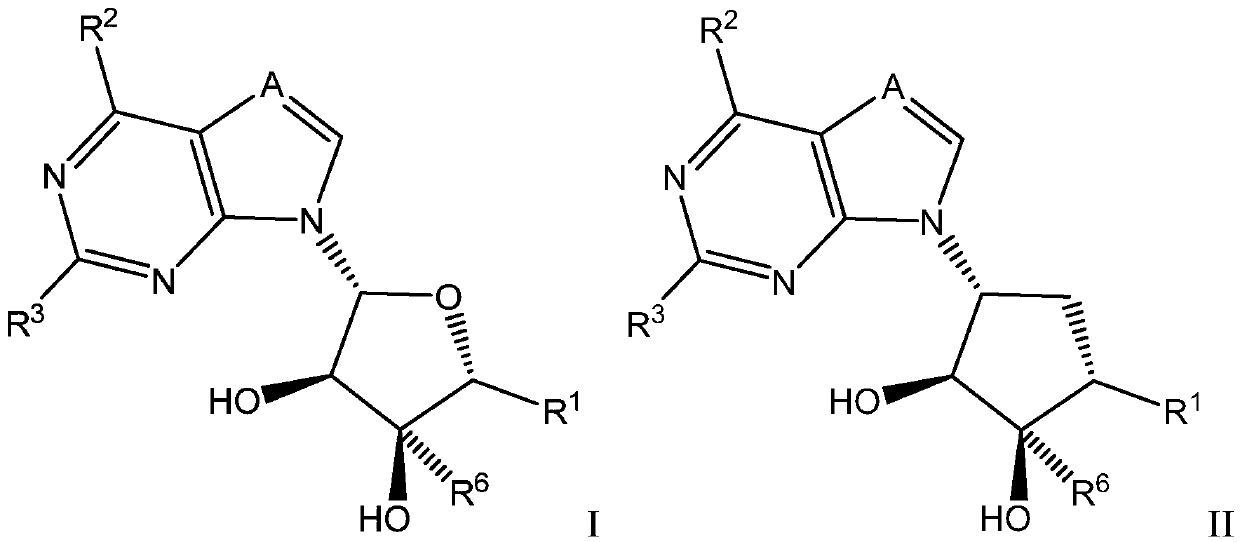
Selective inhibitors of protein arginine methyltransferase 5 (PRMT5)
A methyl and alkyl technology, applied in the field of PRMT5 inhibitors, can solve problems such as gene expression repression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0377] Example 1. (2R,3R,4S,5S)-2-(4-amino-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-5-((R)-1-(bicyclo [4.2.0] Oct-1,3,5-trien-3-yl)-1-hydroxyethyl)tetrahydrofuran-3,4-diol (1)
[0378]
[0379] Step 1. Bicyclo[4.2.0]oct-1,3,5-trien-3-yl((3aS,4S,6R,6aR)-6-(4-chloro-7H-pyrrolo[2,3- d] Synthesis of pyrimidin-7-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methanone (1b)
[0380] A 50 mL RBF with a septum containing magnesium (208 mg, 8.56 mmol) was dried under high vacuum with a heat gun and cooled under Ar. The flask was charged with THF (3.4 mL), 4 / 10 parts of 4-bromobicyclo[4.2.0]oct-1(6), 2,4-triene (1.01 mL, 8.11 mmol) and 1 M diisobutylaluminum hydride in toluene (20 uL, 0.0200 mmol). After stirring for 1 min, magnesium initiation was observed by self-heating of the reaction solution. The reaction mixture was stirred for an additional 10 minutes, diluted with THF (3 mL), and the remaining 4-bromobicyclo[4.2.0]oct-1(6),2,4-triene was charged in two portions over 10 mi...
Embodiment 2
[0391] Example 2. (2R,3R,4S,5R)-2-(4-amino-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-5-((R)-bicyclo[4.2. 0]oct-1(6),2,4-trien-3-yl(hydroxy)methyl)tetrahydrofuran-3,4-diol (2)
[0392]
[0393] Step 1. (R)-bicyclo[4.2.0]oct-1,3,5-trien-3-yl ((3aR,4R,6R,6aR)-6-(4-chloro-7H-pyrrolo[ 2,3-d]pyrimidin-7-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methanol (2a) and (R)-bicyclo[4.2.0]oct-1,3,5-trien-3-yl((3aR,4R,6R,6aR)-6-(4-chloro-7H-pyrrolo[2,3 Synthesis of -d]pyrimidin-7-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methanol (2b)
[0394] Under argon will contain [(3aR,4R,6S,6aS)-4-(4-chloropyrrolo[2,3-d]pyrimidin-7-yl)-2,2-dimethyl-3a,4 ,6,6a-tetrahydrofuro[3,4-d][1,3]dioxol-6-yl]-(4-bicyclo[4.2.0]octa-1,3,5-triene A solution of methanone (1200.mg, 2.82mmol) in toluene (25mL) in 100mL RBF with septum was cooled to -76°C in an acetone / dry ice bath. A 1M solution of diisobutylaluminum hydride (DIBAL) in toluene (5.4 mL, 5.4 mmol) was added dropwise over 4 minutes. ...
Embodiment 6
[0400] Example 6. (2R,3R,4S,5R)-2-(4-Amino-5-fluoro-pyrrolo[2,3-d]pyrimidin-7-yl)-5-[(R)-4- Bicyclo[4.2.0]oct-1,3,5-trienyl (hydroxyl)methyl]tetrahydrofuran-3,4-diol hydrochloride (Example 6)
[0401]
[0402] a) (S)-[(3aR,4R,6R,6aR)-4-methoxy-2,2-dimethyl-3a,4,6,6a-tetrahydrofuro[3,4-d][1 ,3]Synthesis of dioxol-6-yl]-(4-bicyclo[4.2.0]oct-1,3,5-trienyl)methanol (6a)
[0403] To a solution of 4-bicyclo[4.2.0]oct-1,3,5-trienylboronic acid (3500.0 mg, 23.65 mmol) in toluene (90 mL) was slowly added diethylzinc (23.74 mL) at 25 °C , 47.48 mmol). The mixture was stirred at 60°C for 1 hour. Slowly add (3aR,4R,6S,6aR)-4-methoxy-2,2-dimethyl-3a,4,6,6a-tetrahydrofuro[3,4-d][1 ,3] Dioxol-6-carbaldehyde (3200.0 mg, 15.83 mmol) in toluene (40 mL). The mixture was stirred at 60°C for 2 hours. TLC (PE / EA=5 / 1) showed the reaction was complete. Water (10ml) was added to quench the reaction. The mixture was filtered. The filtrate was concentrated and passed through CH with 5 / 95 to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com